1000/1000
Hot
Most Recent

Mitsugumin 53 (MG53), a TRIM family protein, plays a key role in repairing cell membrane damage and facilitating tissue regeneration. Clarifying the role of MG53 and its molecular mechanism are important for the application of MG53 in regenerative medicine. In this review, we analyze current research dissecting MG53's function in cell membrane repair and tissue regeneration, and highlight the development of recombinant human MG53 protein as a potential therapeutic agent to repair multiple-organ injuries.
The cell membrane allows a complex communication and exchange between the inside of a cell and its extracellular environment. Within a life time, cells may be injured by different factors including mechanical stress, radiation such as UV light, and biochemical drugs. If the membrane injury is not repaired in time, the injury will progress to cell death and permanent tissue damage. It is critical to maintain cellular integrity to ensure cell survival and tissue regeneration, as defects in cell membrane repair are linked to the pathophysiology of many human diseases including muscular dystrophy, heart failure, lung injury, and kidney disease. As a rapidly developing field, regenerative medicine aims to repair or replace damaged cells, tissues, and organs [1] A potential therapeutic approach targeting cell membrane repair for regenerative medicine is the recently discovered Mitsugumin 53 (MG53).
MG53 is a tripartite motif-containing (TRIM) family protein and plays a key role in repairing cell membrane damage and facilitating tissue regeneration. MG53 was first identified from skeletal muscle using a novel immunoproteomic approach described by Weisleder, Takeshima, and Ma[2]. In 2009, we reported that MG53 acted as a key component of the cell membrane repair machinery, and demonstrated that mice with ablation of MG53 (mg53−/−) display compromised sarcolemma repair with progressive myopathy[3]. MG53 initiates the assembly of repair-patch formation through facilitating the movement of intracellular vesicles to the membrane injury site. In heart studies, mg53−/− mice have shown increased susceptibility to stress-induced myocardial infarction due to impaired cardiomyocyte repair function[4]. Subsequent studies revealed pathology in the kidney, lung, and cornea of mg53−/− mice, which are also linked to defective cell membrane repair function of the affected tissues[5][6][7]. The therapeutic benefits of the recombinant human MG53 (rhMG53) protein in treatment of stress-induced injuries to the skeletal muscle, heart, lung, kidney, cornea, brain, liver, and skin were established in different animal models [5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21] (Table 1).
| Tissues | Phenotype of mg53−/− Mice | Therapeutic Application of rhMG53 Protein |
|---|---|---|
| SkeletalMuscle | muscle repair defect [3] reduced exercise capability [3,8] |
muscle injury associated with exercise [3,9], muscular dystrophy [10] |
| Heart | defective cardiomyocyte repair [4,11] increased vulnerability to myocardial infarction |
acute myocardial injury [12] chronic heart failure |
| Lung | defective alveolar structure and exacerbated lung injury under stress conditions [6] |
acute lung injury, sepsis, chronic obstructive pulmonary disease [6,13,14] |
| Kidney | proximal tubular pathology [5] increased susceptibility to acute kidney injury |
acute kidney injury [5] nephrotoxicity [15], chronic kidney disease |
| Cornea | reduced re-epithelialization and increased fibrosis following alkaline injury [7] | cornea injury and ulceration [7] prevention of cornea fibrosis |
MG53 is predominantly expressed in striated muscles [3]. Studies show that low levels of MG53 are also present in the lung epithelial cells, inner cortex of the kidney, along within the tear film, corneal epithelia, and aqueous humor[5][6][7][13][14][18]. MG53 can be secreted from skeletal muscle and circulates throughout the entire body to reach all tissues and organs[22][23][24]. This protein is highly conserved in many other species in the animal kingdom, which preserves its fundamental and universal biology functions [3].
More than 80 known TRIM protein genes have been identified in humans so far, which all share the RING B-box coiled-coil (RBCC) motif. The RBCC domain comprises three motifs which are a RING-finger domain, one or two B-box domains, and a coiled-coil domain. Their cellular functions are diverse including cell proliferation, differentiation, development, oncogenesis, apoptosis, protein quality control, autophagy, innate immunity, and retroviral replication [25][26][27][28][29][30]. Genomic analysis of the TRIM family reveals that the human TRIM family is split into two groups that differ in domain structure, genomic organization, and evolutionary properties. MG53 belongs to group 2, which is characterized by the presence of a carboxyl-terminal SPRY domain, a repeated sequence in the dual-specificity kinase SplA, Ca2+-release channel ryanodine receptors (RyR), and a unique set of genes in each mammal examined[31].
Molecular phylogenetic analyses reveal that MG53 forms a close association among other organisms (Figure 1A). The human MG53 shares homology with lots of mammalian, especially primates including orangutan/Pongo abelii (99.6%), gorila/Troglodytes gorilla (99.4%) and Tcardiol/Hylobates moloch (99.2%). The homology indicates that diverse animals with the MG53 gene have a significant close evolutionary relationship that might have originated from a common ancestor.
Figure 1. (A). Molecular phylogenetic analysis of MG53 proteins in different species by maximum likelihood. The evolutionary tree is presented to compare MG53 in different species. The maximum likelihood method and JTT matrix-based model were applied for inferring the evolutionary history[32]. The tree with the highest log likelihood (−7643.24) is shown. Neighbor-join and BioNJ algorithms were applied to a matrix of pairwise distances estimated using the JTT model, and the topology with superior log likelihood value was selected, then the initial tree(s) for the heuristic search were obtained. This analysis involved 95 amino acid sequences. There were a total of 599 positions in the final dataset. Evolutionary analyses were conducted in MEGA X[33]. (B). The primary amino acid sequence of MG53 contains the following characteristic tri-partite motifs (TRIM): RING, B-Box and Coiled-coil motifs in the amino terminus, and the PRY and SPRY domains at the carboxyl terminus. C242 is a critical cysteine residue involves in redox-dependent oligomerization of MG53 associated with cell membrane repair.
Based on the phylogenic tree, MG53 proteins are divided into two categories according to the variants of amino acids in different domains, such as RING finger, B-box zinc, and SPRY domain (Figure 1B and Table 2). Based on their functional domains, MG53 homology can be grouped into two categories. The first category, represented by human MG53 protein, consists principally of three functional domains: RING finger, B-box zinc, and SPRY domain. Members of the second category, including mouse and rat, contain additional domains such as Poly (hydroxyalcanoate) granule-associated protein (phasin) and SPRY-associated domain (PRY). The RING finger domain mediating ubiquitination is the characteristic signature of E3 ubiquitin ligase and the zinc-finger motif of the MG53 through binding two zinc cations to Cys3HisCys4 amino acid. However, the specific function of the B-box domain remains unclear. In general, the PRY-SPRY domain is recognized as more evolutionarily ancient, which conveys the selectivity and specificity of its E3 activity[34][35]. Mutations found in the SPRY-containing proteins can cause Mediterranean fever and Opitz syndrome[34][35].
| MG53 Species | Gene ID | Amino Acid (aa) | Identities (%) | Identities (%) | Protein Structure | ||
|---|---|---|---|---|---|---|---|
| RING | B-Box | SPRY | |||||
| Homo Sapiens | NP_001008275.2 | 477 | 100 | 100 | 100 | 100 |  |
| Pan Troglodytes | XP_001157628.2 | 477 | 100 | 100 | 100 | 100 |  |
| Macaca Mulatta (Isoform 2) |
XP_001112866.1 | 477 | 98.95 | 100 | 100 | 97.93 |  |
| Canis Lupus Familiaris (Isoform X2) | XP_005621293.1 | 477 | 93.50 | 95.74 | 100 | 89.12 |  |
| Bos Taurus | XP_002698119.1 | 482 | 94.71 | 95.74 | 100 | 90.16 | 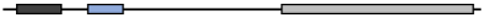 |
| Mus Musculus | NP_001073401.1 | 477 | 91.19 | 95.74 | 97.56 | 87.56 |  |
| Rattus Norvegicus | NP_001071143.1 | 477 | 90.78 | 93.62 | 97.56 | 86.53 |  |
| Xenopus Tropicalis | NP_001008188.1 | 477 | 59.14 | 53.19 | 72.50 | 62.63 | 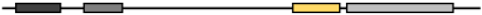 |
 RING finger
RING finger  B-box zinc finger
B-box zinc finger  B-Box-type zinc finger
B-Box-type zinc finger  SPRY domain
SPRY domain  Poly(hydroxyalcanoate) granule associated protein (phasin)
Poly(hydroxyalcanoate) granule associated protein (phasin)  SPRY-associated domain (PRY).
SPRY-associated domain (PRY).
MG53 is highly conserved among different species, including human, monkey, rat, and mouse[3][34][35] The RING finger motif that contains a Cys3HisCys4 amino acid motif is a zinc-finger motif binding with two Zn2+ [3]. The B-box domain is another zinc-binding motif, and its specific function may be linked to cell membrane repair and wound healing [36][37]. Coiled-coil domains mediate homo- or hetero-oligomerization of TRIM family proteins, especially for their self-associations and interactions with different binding partners[37]. The PRY-SPRY domain is believed to be a central mediator for selective interaction with its partners[38]. A critical cystidine residue (C242) in the PRY-SPRY domain is critical for MG53 oligomer formation to facilitate dysferlin or annexin fusion vesicles at the membrane injury sites[3][36][37].
MG53 is abundantly distributed in mouse and rat skeletal muscle and heart, but its expression level is very low in hearts of human, porcine, and[35] species along with no expression in liver, skin, and brain[3][9][21]. MG53 also locates in the kidney and lung with much lower expression than that in heart and skeletal muscle[5][6][13][14]. Native MG53 distributed in the corneal epithelia, tear film, and aqueous humor suggests its potential function in corneal homeostasis[7][24]. Interestingly, MG53 can be secreted from the muscle into the blood circulation and participates in multiple physiologic and pathologic processes, in particular for membrane repair in non-muscle organs[7][9][19][21].
To expand the mechanistic evaluation of MG53 in cell membrane repair and tissue regeneration, we have created transgenic mice with constitutive secretion of MG53 in the blood stream (tPA-MG53) [9]. The tPA-MG53 mice lived a healthy life-span. These mice exhibited significant enhanced capacity of tissue regeneration without alteration of glucose handling in metabolism[9].
Striated muscle cells undergo severe membrane stress in response to muscle contraction, so acute muscle membrane repair is particularly important[39]. Several molecular components such as dysferlin and CaV3 were identified in its repair, particularly those specific to cardiac and skeletal muscles[40][41][42][43][44]. Cai et al. first reported that MG53 was a central component of the plasma membrane repair machinery and facilitates repair of acute membrane damage in an oxidation-dependent manner[3][45][46]. Genetic ablation of MG53 in mice (mg53−/−) resulted in defective membrane repair function in striated muscle and led to progressive skeletal myopathy [3].
The function for MG53 to repair acute cell membrane injury is illustrated in Figure 2, where red fluorescent protein (RFP)-labeled MG53 was transiently expressed in C2C12 cells. Prior to injury and at resting state, RFP-MG53 was present on the intracellular vesicles, plasma membrane, and in the cytosol. Poking of the cell by a microelectrode resulted in rapid translocation of RFP-MG53 containing vesicles to the acute injury site. Injury of the cell led to transient change of the redox state from reduced environment to oxidized environment, which triggered redox-dependent oligomerization of MG53 allowing for formation of the membrane repair patch[3][47].
Figure 2. RFP-MG53 expressed in C2C12 cells moved quickly to the acute injury site following mechanical poking of the cell. Image shown on the right was taken at 60 s after injury. For more details, see Ref.[3][36][37]
The MG53-mediated membrane repair process is regulated by multiple factors. Zn2+ can interact with MG53 to promote cell membrane repair because both RING and B-box motifs of MG53 have Zn2+-binding domains which contribute to MG53-mediated repair [36]. Leucine zipper motifs in the coiled-coil domain of MG53 enrich understanding of the mechanism that facilitates oligomerization of MG53 during membrane repair. Both oxidation of the thiol group of Cys242 and leucine zipper-mediated interaction among the MG53 molecules contribute to the nucleation process for MG53-mediated membrane repair [37]. Extracellular Ca2+ facilitates repair vesicles’ fusion to reseal the membrane [3].
Vesicle-related proteins are involved in the regulation of the MG53-mediated membrane repair process. Polymerase I and transcript release factor (PTRF) acts as a docking protein leading MG53 to the membrane during the repair via possible binding to exposed membrane cholesterol at the injury site. Specific RNA silencing of PTRF leads to defective muscle membrane repair, and overexpression of PTRF can rescue membrane repair defects in dystrophic muscle[48]. Furthermore, as a key cytoskeleton motor protein, non-muscle myosin type IIA (NM-IIA) interacting with MG53 essentially regulates vesicle trafficking [49], along with interactions of MG53 with both dysferlin and CaV3 during cell membrane repair [50][8,50]. Dysferlin and annexin A1, along with MG53, play direct roles at sites of damaged membrane for remodeling of the transverse tubule system, whereas CaV3 indirectly affects membrane resealing because of altered trafficking[51].
Some amino acids on the MG53 primary sequence are required for MG53-mediated membrane repair. A single amino acid replacement, K279A, leads to severe aggregation of MG53 within inclusion bodies in HeLa cells. Due to the loss of the positive charge, the localization and function of dysferlin and MG53 are significantly changed [52]. Recently, MG53-associated membrane repair was linked to a mono-ADP-ribosylation in wound healing following myocardial injury. ADP-ribosylation interfered with assembly of MG53 repair complexes in MG53 R207K and R260K mutations[53]. As the target of S-nitrosylation (SNO) and oxidation SNO, with C144 mutation, MG53 C144S prevented oxidation from oxidation-induced degradation of MG53, which resulted in its preservation of the protein and enhanced cell survival following oxidative insult[54][55].