Focus on the Small Intestine
The small intestine is anatomically divided into three regions whose proportions are as follows: duodenum, which in adult pig represents about 4–4.5%, jejunum, about 88–91% and finally ileum, about 4–5%
[3]. The newborn has similar proportions to those of the adult, even if the differentiation between jejunum and ileum is not well defined. Although in adults, these three traits have morphological characteristics that clearly distinguish them, they also share many common features as they are all organized in four basic layers: the tunica serosa, the tunica muscularis, the tunica submucosa, and the tunica mucosa. The serosa is the outer layer and morphologically consists of an epithelium lining on connective tissue, which contains blood vessels and nerves, and which eventually forms the mesentery. The tunica muscularis is organized with two distinct layers of muscle fibers; an outer layer of longitudinal fibers and an inner layer of circular fibers, which both are involved in GIT motility. The submucosa is made up of a layer of connective tissue that contains blood and lymphatic vessels and nerves.
A special focus should be done on the submucosa of the duodenum, because it contains the Brunner’s glands, which are specialized to produce alkaline secretion containing bicarbonate, in order to protect the duodenal mucosa from the acidic content of the chyme coming from the stomach; in addition, this secretion stimulates the activation of intestinal enzymes by creating an alkaline environment and lubricating its walls. Nutrition, nervous and/or hormonal stimuli and reflexes increase the secretory activity of Brunner’s glands
[4].
The inner layer, the mucosa, is the one that characterizes the morpho-functional activity of each individual trait of the intestine and it is made up of three sublayers: the muscularis mucosa, the lamina propria and the epithelium. The muscularis mucosa is a thin layer of muscle fibers that divides the submucosa from the mucosa and that contributes to form the transient intestinal folds. The lamina propria is made up of connective tissue that contains blood vessels, neurons, lymphocytes and, in the ileum, it also contains lymph nodes called Peyer’s patches; the lamina propria has the duty of sustaining the avascular epithelial layer. This latter consists of a single layer of epithelial cells, which covers the luminal surface of the intestine.
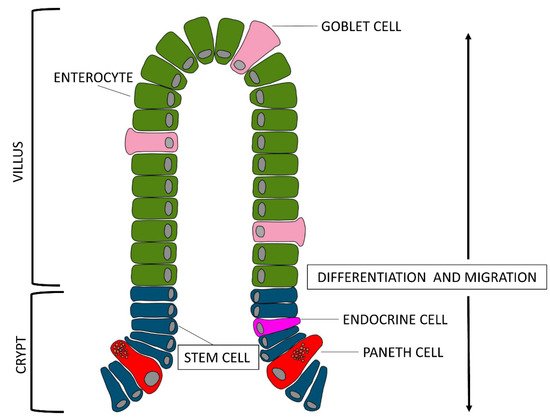
The mucosa as a whole, folds back to form finger-like projections called villi, and at the base of these ones, the Crypts of Lieberkühn, also known as intestinal glands, which are moat-like invaginations.
Villi increase the work surface by at least five times compared to a flat surface of the same size. Furthermore, the luminal surface of the enterocytes has got microvilli at the level of the apical membrane (also called brush border), which further increase the absorptive surface by 15–40 times, simulating the observed system of the villi folds. Microvilli are placed in a gelatinous layer of glycoproteins, the glycocalyx, and have digestive enzymes. The villi morphology changes according to the intestinal tracts and this trend reflects their different functions: the length increases from the duodenum to the mid-jejunum and then decreases again towards the distal ileum. Similarly, the crypts also change in size and composition along the intestine: they are deeper in the duodenum and jejunum and less deep in the ileum.
[5].
If we look at the epithelial layer more closely, we notice that there are three types of cells lining on the villus surface: enterocytes, goblet cells, and enteroendocrine cells (piglet gut barrier reviewed by
[6]). Enterocytes are the most abundant group of villi cells, about 94%, goblet cells are about 5% and endocrine cells about 1%. All of these cell types originate from stem cells located at the base of the crypts
[7]. Enterocytes get mature while they migrate from the base to the tip of the villi; their enzymatic activity, which reflects the digestive function, begins when the enterocytes reach the basal third of the villi axis, while their absorption function begins when enterocytes reach the medium-high level and spreads its maximum when the cells reach the top of the villi. Obviously, the enterocytes present on the surface of the villi are continuously renewed.
The goblet cells are secreting cells: specifically, they secrete viscous mucus and are located between the enterocytes. They increase in number from the proximal portion of the jejunum to the distal one of the ileum. Goblet cells secrete relentlessly viscous mucus and their basal activity increases when the cells come in contact with a secretagogue substance. Mucus production can be altered by several agents: cholinergic agents, neuropeptides, hormones, and toxins for example. Furthermore, nutrients are capable of influencing the thickness and production of mucus (e.g., milk peptides). In addition, the enterocytes that organize the mucosa can also have secreting activity contributing to produce, with the mucus, the intestinal juice: a young pig of about 70 kg, produces approximately 6 L per day
[3]. The third cell type, the enteroendocrine cells, produce hormones to regulate the functionality of the gastrointestinal tract
[8].
Interesting, recent works suggest the presence in the GIT of pig of another type of cells, the Paneth cells, which have been described extensively in other species
[9]. Reference
[10] observed these cells, located adjacent to stem cells at the bottom of the crypt of the ileum, and
[11] observed Paneth cell-like cells in the gut of piglet from newborn to weaned stages in the same localization. The exact function is unknown, but due to the presence of substances, such as lysozymes and defensins inside microgranules of the cytoplasm, they most likely contribute to maintenance of the gastrointestinal barrier. About the differentiation of Paneth cells, it would be possible to hypothesize that, as described for other species, they move back to the stem cell compartment to be interspersed with stem cells
[12].