1000/1000
Hot
Most Recent

Permanent grasslands are main habitats for many plant species and pollinators. Their destruction as well as their intensification has a major impact on plant and pollinator biodiversity, which has a cascading effect on pollination. However, we lack an understanding of these effects, thereby limiting our ability to predict them. In this review, we synthesised the literature on the mechanisms behind this cascade to provide new insights into the relationship between land-use intensification and pollination. By matching functional traits that mediate the relationship between the two trophic levels, we identified major knowledge gaps about how land-use intensification affects plant–pollinator interactions and how it favours plants with generalised floral traits, which are likely harmful to pollination.
Insect pollination on permanent grasslands relies on interactions between flowers and pollinators (hereafter, “plant–pollinator interactions”). An approach that includes the morphological, physiological and phenological features of organisms that affect their fitness [1] is useful because plant and pollinator features together drive plant–pollinator interactions. These functional features, called “matching traits” [2] mediate relationships between the two trophic levels [3]. Several plant traits (hereafter, “floral traits”) and pollinator-matching traits are involved in plant–pollinator interactions (Table 1). For example, flowers with deep corollas can only be accessed by pollinators with long mouthparts. Matching trait values can be calculated at the community scale, and the community weighted mean (CWM) is the mean value of traits weighted by the abundance of each species in a community. Functional diversity (FD) is the value, range, and relative abundance of functional traits in a given community [4]. In the mass-ratio hypothesis, an ecosystem’s functions depend on the CWM [4]. The hypothesis of niche complementarity suggests that greater FD values increase niche partitioning and lead to species complementary, which serves the ecosystem functions [5]. These hypotheses have been extensively tested for vegetative functional traits but much less so for the relationships between floral traits and pollination.
| Matching Traits Categories |
Matching Traits | Function | Agricultural Practices or Land-Use Index |
Effect | Number of Grasslands | Knowledge Level | Country | References |
|---|---|---|---|---|---|---|---|---|
| Signals | Allow communication between plants and pollinators and thus interaction between them. Signals generate sensory experiences for pollinators that are different from an animal species to another | [6][7] | ||||||
| Colour (hue) | Detection from background [8] | LUI | Shift toward white | 69 | T—D | Germany | [9] | |
| Photoreceptors and visual system | Matching level between visual system and colour |
LUI | - | 119 | NT—I | Germany | [10] | |
| VOC emitted | Detection of flower [6] | Grazing and fertilization | None | 2 | T—D | France | [11] | |
| ND | Odour preferences | Not tested | ND | NT—I | ||||
| Exploitation Barrier | Prohibit interaction with a pollinator if its own matching traits are not adapted | [12] | ||||||
| Nectar tube depth | Threshold to be reached by pollinator mouthpart length [13] |
LUI | - | 40 | NT—D | Germany | [14][15] | |
| Relative proboscis length | Depth of exploitable flowers | LUI | - | 40 | T—D | Germany | [16] | |
| Rewards | Essential food for pollinators. They gather mainly nectar as source of carbohydrates and pollen as source of proteins. Rewards are linked with pollinator matching traits which inform for instance on their food needs | [17][18][19] | ||||||
| Nectar production | Total quantity of sugar in a grassland [20] |
Nitrogen deposition | - | 768 | T—I | Great-Britain | [21] | |
| Livestock Unit/ha/year | - | 561 | T—D | Scotland | [22] | |||
| Pollen production | Total quantity of pollen in a grassland | LUI | - | 119 | T—I | Germany | [23][24] | |
| Livestock Unit/ha/year | - | 561 | T—D | Scotland | [22] | |||
| Body size | Quantity of pollinator food needs | LUI | - | 40 | T—D | Germany | [16] | |
| Phenology | Temporal availability of rewards [25] | Mowing, grazing, fertilization | (i.e., advances) or none | 33 | T—D | France | [26] | |
| Livestock Unit/ha/year | 561 | T—D | Scotland | [22] | ||||
| Sociability level | Duration of the breeding period | Not tested | - | NT—I | ||||
| Nectar sugar concentration and nectar viscosity | Nectar feeding rate [27] | Not tested | + | NT—I | ||||
| Anatomy of mouthpart | Adaptation to liquid viscosity | LUI | Shift toward sponging-sucking | 40 | NT—I | Germany | [16][28] | |
| Pollen amino acid concentration and protein content | Pollen quality [29] | LUI | - | 40 | NT—I | Germany | [15] | |
| Pollinator stoichiometric niche | Quality of pollinator food needs | Not tested | - | NT—I |
The generalisation of floral traits mainly affects the accessibility of floral rewards (pollen, nectar) for pollinators. However, it may also reflect a decrease in the quality of these rewards since many plants favoured by intensification belong to the family Asteraceae [23], which often has low pollen quality [30]. Intensification may also decrease rewards production on grasslands by reducing in the total plant cover that produces rewards and by favouring wind-pollinated grasses [31]. Overall, these changes could have a major impact on pollination, as decreases in floral rewards quantity and quality is a major threat to pollinators [20].
Intensification could also change pollinator community composition. First, total abundance of pollinators, which provides quantitative information about pollination, may fall due to the lower (reward) attractiveness of the grassland and lower food availability. Second, intensification is expected to lead to a decrease in the mean values of pollination effect traits [32], which provide information about the effects of organisms on ecosystem functions [33] (i.e., qualitative information about pollination). Even though traits such as pollinator body size have not been found to be relevant in all ecosystems [34], they are essential to understanding the qualitative differences among pollinators both mechanistically and functionally [35]. Here, we aim to consider both quantitative and qualitative components of pollination because they are rarely considered together despite their high complementarity [2].
Figure 1 illustrates the cascading effects from land-use intensification on pollination highlighted in this review. It shows that land-use intensification influenced the FD and CWM of floral traits (step 1), which in turn influenced the FD and CWM of pollinator matching traits (step 2), thereby affecting both quantitative and qualitative components of pollination (step 3).
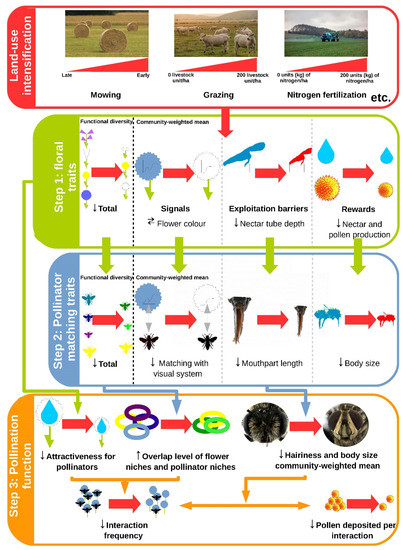
Intensification can be associated with (i) a shift in the dominant colour of flowers (as perceived by Apis mellifera ) from blue or yellow to white at the community level and (ii) a decrease in flower colour diversity, but only when measured before the first mowing as recorded in two German regions, [9]. Phylogenetic clustering does not explain this result despite a relationship between flower colour and phylogeny [9]. Pollinators prefer certain colours, due in part to their photoreceptors [36]. For instance, Diptera may be more abundant on grassland plots with either yellow or white flowers, depending on their preferences [19]. As the visual spectrum of insects often extends into the ultraviolet, most pollinators can detect white [37]. Pollinators can also learn to detect other colours, even though the limited learning capacities of Diptera can restrict their shifts toward a different colour [8]. Overall, even though intensification may lead to a higher relative abundance of white flowers, [10] it suggests a matching disruption between flower colour and the visual system of pollinators when intensification is high. Hence, the influence of flower colour on pollinator assemblage remains unclear.
However, little information is available on relationships between pollinator matching traits and flower odour traits. Two traits influence a pollinator’s ability to recognise scents: the length of the antennae that bears odorant sensilla and the number of odorant receptor types [38]. For instance, longer antennae may have more receptors, which would increase the ability to detect odours and rely on odour signals or cues to interact with flowers [39]. However, these traits do not provide clues about the flower scent preferences of pollinators. Hence, future studies into the influence of grassland intensification on the relationship between odourscape and pollinator attraction are needed.
A decrease in rewards production may prevent certain pollinators from meeting their high metabolic requirements. [16] observed that the CWM body size of pollinators—which positively correlates with the metabolic rate in arthropods [40]––was twice as large on less intensive grasslands than on the most intensive grasslands. This result is partly explained by an increase in the relative abundance of Diptera, which are on average smaller than bees, according to [15]. One can also expect a decrease in the abundance of pollinators such as large or social bees because they require much more pollen to raise larvae or develop the colony [41] compared to Diptera, which has free-living larvae and a strong ability to store protein to produce eggs [42]. By decreasing plant species richness, intensification may also decrease the temporal stability of flower resources, thus mainly affecting pollinators that need nectar and pollen throughout the season [43]. This is the case for bumblebees, which cannot store large quantities of pollen [30], and also for most social and multivoltine bee species. Pollinators with a short period of activity may also be disadvantaged by intensification if they face a resource shortage when they emerge [44]. To confirm these assumptions, studies are needed on the relationships between intensification and pollinator metabolic requirements on grasslands.
One way to study the relationships between ecosystem functioning and plant–pollinator interactions is to analyse the latter’s degree of specialisation. Indeed, the more an interaction network is specialised, the higher the complementarity of its interactions and the differentiation of species niches [45]. An increase in complementarity implies that more functionally complementary species are needed to fulfil the ecosystem function [46]. Matching traits are useful for describing the niches of plants and pollinators [47] and providing mechanistic explanations for the degree of complementarity of plant–pollinator interactions. [48] showed that a plant community with higher floral diversity had higher plant–pollinator interaction network complementarity (measured by H2′, an index that describes the complementarity of interaction, [49]). In our review, we suggest that the CWM of nectar tube depth may decrease with intensification. Hence, flowers may be exploitable by a larger pool of pollinators, which reflects a plant community with more generalised exploitation-barrier traits. Moreover, intensification decreases forb richness [31] and thus may likely decrease flower functional diversity [50] due to its positive relationship with taxonomic diversity. Hence, intensification should generate networks with low functional complementary because of high niche overlap in floral traits among plant species. However, [23] found that intensification decreased plant species diversity but did not decreased H2′, which remained high overall. [51] observed the same lack of correlation without looking at the effect of intensification.
The degree of network specialisation may be explained in part by the matching traits but also by other processes, such as resource competition between pollinators. Hence, two competing pollinators with the same matching traits values may lead to fidelity for a flower [52] that they match less well. This highlights the need to define specialisation of plant–pollinator interactions carefully [53]. However, on intensively managed grasslands, despite the loss of pollinator species, the stability of pollination function loss may increase, because pollinators are more interchangeable than on less intensive grasslands.
Intensification is likely to decrease the flower functional diversity (e.g., flower colour FD in [9]). Two assumptions can be made concerning the relationship between the FD of floral traits and interaction frequency. First, this relationship may be negative because a higher FD may blur the visual signal, leading to an increase in search time (serial processing; [54]). This assumption was confirmed in the studies of [50] and [55], which recorded a low taxonomic diversity of pollinators with a few generalist pollinator species representing most of interactions. Secondly, we expected a positive relationship between the functional diversity of floral traits and interaction frequency due to a better distribution of pollinators and a greater complementarity of pollinator niches [46]. Ref. [56] confirmed this hypothesis on permanent grasslands with 247 pollinator species. The highly diverse pollinator community recorded in this study may have increased the interaction frequency and the complementary between pollinator niches. Hence, more studies are needed to understand how floral trait functional diversity affects interaction frequency, and to confront niche theory with cognitive ecology, as the latter is based mostly on experiments performed under non-natural conditions [57]. Lastly, to improve understanding of how niche complementarity shapes the relations between floral functional diversity and interaction frequency, studies that include functional indices on each component of functional diversity (e.g., functional evenness, functional richness, functional divergence; [5]), not aggregative indices like functional entropy, Ref. [58] are needed.
Besides interaction frequency, information about the quality of interactions is needed [2][35]. Quality per interaction is often measured as the quantity of pollen deposited by a pollinator during a single visit to a freshly opened flower. This seems to be positively correlated with pollinator hairiness [59][60]. However, these two studies only focused on three cultivated plants species with easy access to the reproductive organs. Ref. [61] showed that pollinators’ facial pollen load increased with facial area and hairiness on 127 bee and fly species and 36 wild plants. Ref. [16] found that intensification led to a decrease in the CWM of both relative hairiness and body size of pollinators. An increase in the relative abundance of Diptera, which are less hairy [16][61] and smaller than bees [15] and have different pollination behaviour [62] may explain this result. This shift in pollinator community highlights the need to consider the phylogenetic signals between pollinator effect traits such as hairiness, body size and behaviour, and their respective effects independently.