Thiophene derivatives provide useful intermediaries in various areas of science and industry, with a wide range of applications, and therapeutic properties. Thiophene derivatives attract both great academic interest, and interest from the agrochemical, pharmaceutical, and dye industries, as well. As to their biological and pharmacological applications, thiophene derivatives possess remarkable properties as antipsychotic, antianxiety, antifungal, antimicrobial, antioxidant, anticancer, and anti-inflammatory agents. The present work provides an update on the role of thiophene-based derivatives in inflammation processes.
1. Introduction
Inflammation is a tightly and carefully regulated, protective process, both complex and multifactorial, and mounted by the innate immune system in response to harmful stimuli such as ischemia, tissue damage, autoimmune injuries, dead cells, pathogens, toxins, and chemicals. The mechanisms involved in this process are characterized by a complex series of events that involve changes in vascular permeability, exudation of fluids containing plasma proteins, and migration cells within the immune system, such as leukocytes, lymphocytes, and macrophages into the inflammatory area
[1][2][3][4][5].
These mechanisms are mediated through a great variety of soluble micromolecules, which include several secreted polypeptides known as cytokines. Inflammatory cytokines can be classified and are involved in both acute and chronic inflammation. In accordance with the cellular microenvironment, they may present either pro- (Th1) or anti- (Th2) inflammatory activities. The most common anti-inflammatory cytokines are interleukins IL-4, IL-10, IL-13, and TGFβ (transforming growth factor). The most common proinflammatory cytokines are tumor necrosis factor (TNF), and interleukins IL-1, IL-2, IL-6 and IL-7
[5][6][7][8].
To maintain or to re-establish homeostasis, proinflammatory cytokine control, production, and regulation are essential. Controlled proinflammatory cytokine production helps contain the inflammatory process and reduce tissue damage. However, excess production exacerbates the inflammatory process, in many cases generating the onset of chronic inflammatory disease
[7][9][10].
Heterocyclic compounds have historically played an important role in the search for bioactive products. It is observed that more than 75% of drugs in clinical use have at least one heterocyclic ring in their chemical structure
[11]. Thiophene and its substituted derivatives, all heterocyclic compounds, have been our focus of interest for almost two decades.
Thiophene derivatives provide useful intermediaries in various areas of science and industry, with a wide range of applications, and therapeutic properties. Thiophene derivatives attract both great academic interest, and interest from the agrochemical, pharmaceutical, and dye industries, as well
[5][12][13]. As to their biological and pharmacological applications, thiophene derivatives possess remarkable properties as antipsychotic, antianxiety, antifungal, antimicrobial, antioxidant, anticancer, and anti-inflammatory agents
[5][12][14][15][16][17]. Many marketed drugs, such as Olanzapine, Benzocyclidine, Sertaconazole, Tioconazol, Dorzolamide, Tipepidine, Ticlopidine, Clopidogrel, Pasugrel, Citizolam, Timepidium and Tiquizium Bromide contain a thiophene moiety.
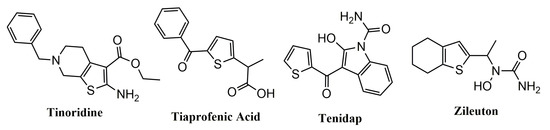
Tinoridine, Tiaprofenic acid, Tenidap, and Zileuton (
Figure 1) are the best-known examples of commercially available drugs with anti-inflammatory properties that contain a thiophene ring as pharmacophoric group. The first three are NSAIDs used in the treatment of pain and inflammation. Tinoridine and Tiaprofenic acid act by inhibiting COX enzymes
[14][18], and Tinoridine presents potent antiperoxidative and radical scavenger activity
[18], while Zileuton is a LOX inhibitor
[19].
Figure 1. Chemical structures of market anti-inflammatory drugs containing a thiophene moiety (Tinoridine, Tiaprofenic acid, Tenidap and Zileuton.).
Based on these initial considerations, the aim of this review is to present an update over the last 10 years of the role of thiophene derivatives in inflammation, identify the most promising compounds and anti-inflammatory substitution patterns, and to help direct the synthesis of potentially more active new derivatives. To compose the database, the authors performed a systematic search in PubMed, Capes Journal Portal, ScieELO and Medline databases. No language restrictions were applied.
2. The Role of Thiophene Derivatives in Inflammation
2.1. Thiophene-Based Compounds Inhibitors of COX and/or LOX Enzymes
Cyclooxygenase enzymes (COX) can be found in three isoforms. COX-1, also known as constitutive, is present in normal tissues and produces prostaglandins from arachidonic acid, regulating functions such as gastric mucosa production and platelet adhesiveness
[20][21]. COX-2 is present in certain tissues like the uterus, kidneys and prostate. It is an inducible enzyme, and its levels increase in case of tissue damage, such as inflammation
[22]. COX-3 was discovered in 2002 and is found in the central nervous system. It may be linked to the antipyretic effect of paracetamol, but its function is still not completely understood
[23][24][25].
NSAIDs (non-steroidal anti-inflammatory drugs) were developed with the aim of inhibiting COX activity, but the degree of inhibition of each isoform, i.e., COX-1 or COX-2, can vary and determines side effect profiles
[25][26]. Despite the development of selective NSAIDs for COX-2, many side effects have been observed, especially in situations involving chronic use. Furthermore, there are still controversies about the physiological role of this enzyme
[26].
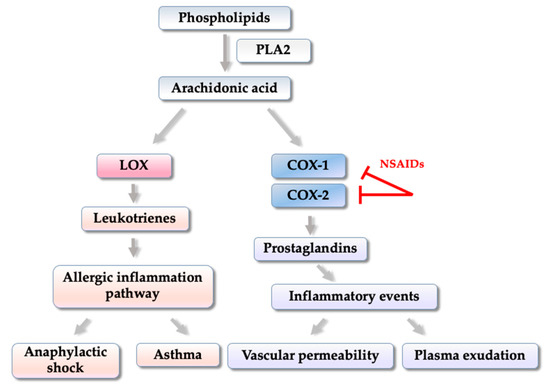
In inflammatory processes, activation of COX-2, and the production of prostaglandins is directly involved in inflammatory events such as increased local blood flow, increased vascular permeability, and plasma exudation (
Figure 2)
[27][28].
Figure 2. Simplified diagram of COX-1, COX-2 and LOX enzymes activation and beginning of the inflammatory process.
2.2. Thiophene Derivatives That Modulate Gene Expression and/or Inflammatory Cytokines
Cytokines are protein molecules produced by many cell types upon antigenic stimulus that carry stimulatory, modulatory or even inhibitory signals to different immune system cells. They can act either in the cell that produced them (autocrine), in nearby cells (paracrine), or in distant cells with the aid of the bloodstream (endocrine)
[29].
Cytokines are important for generating an inflammatory response at infected and/or injured sites. They can regulate gene transcription of other cytokines and stimulate increased production through signaling cascades by second messengers. The result of this stimulus may lead to formation of cytokines that increase (pro-inflammatory) or attenuate (anti-inflammatory) inflammatory processes. We have noted both pro-inflammatory: interleukins (IL) 1, 2, 6, 7, and TNF (tumor necrosis factor), and anti-inflammatory IL-4, IL-10, IL-13 and TGFβ (transforming growth factor β) cytokines
[30][31].
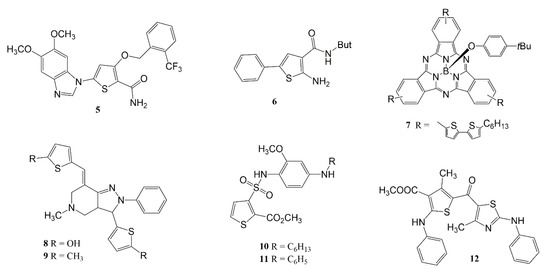
For years, researchers have been studying thiophene derivatives with anti-inflammatory activity and their mechanisms of action. Hu et al.
[32] evaluated methoxy-substituted thiophene derivatives (compound
5) (
Figure 3). After LPS-induced inflammation tests with THP-1 monocytes, it was observed that compound
5 was able to negatively regulate the expression of TNF-α and IL-8, and also inhibit activation of ERK, p38, and NF-ĸB (at 10 µM)
[32].
Figure 3. Chemical structures of thiophene-based compounds, which modulate gene expression and/or inflammatory cytokines.
2.3. Thiophene Derivatives with In Vivo Anti-Inflammatory Activity in Classic Models of Inflammation
Carrageenan-induced paw edema is a classic model of inflammation. The model allows observing inflammatory parameters related to neutrophil activation, pro-inflammatory mediators release (such as IL-6, IL-1β, and TNF-α), and enzymes linked to inflammation such as COX-2
[33]. Carrageenan-induced paw edema involves three stages. The first can be seen at the beginning of the inflammatory process in the first 90 min, where there is the release of histamine and serotonin, responsible for vasodilation and increased vascular permeability. In the second stage, which occurs between 90 and 150 minutes, there is biosynthesis of prostacyclins and other inflammatory process mediators. In the third stage, which occurs after 150 min, prostaglandins are synthesized in the inflamed tissue and leukocyte infiltration commences
[34].
Another widely used model is ovalbumin induced asthma, this consists of triggering a classic cellular response via T helper 2 (Th2) cells and the release of inflammatory interleukins (IL) along with macrophages, eosinophils, and mast cells
[35][36].
Some years later, Kumar et al.
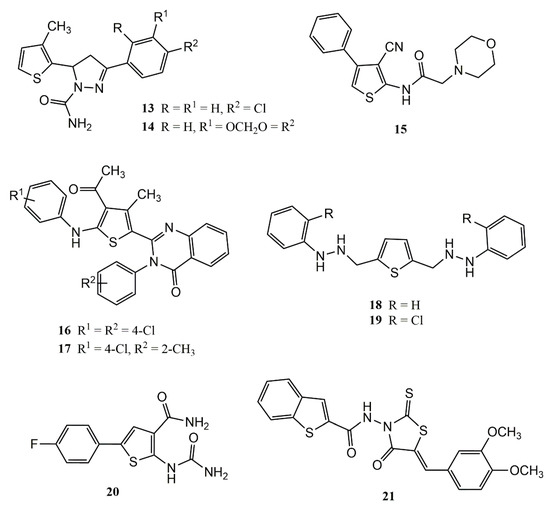
[37] performed in vitro tests with a series of thiophene derivatives and found that the presence of chlorine and methyl groups in the chemical structures, as observed with compounds
13 and
14 (
Figure 4) is fundamental for significant PLA2 inhibition.
Figure 4. Chemical structures of thiophene-based compounds with anti-inflammatory properties in classic models of inflammation.
In a carrageenan-induced paw edema model, the study authors observed that compound
15 (
Figure 4), at a dose of 50 mg/kg, presented anti-inflammatory-inhibition activity (58.46%) superior to indomethacin (47.73%). The authors associated this activity with the morphine ring coupled at the 2-amino position of the thiophene ring
[38].
Compounds
16 and
17, thiophenic derivatives both presenting methyl and chlorine substituents displayed anti-inflammatory activity comparable to sodium diclofenac in a model of paw edema induced by carrageenan (
Figure 4). These derivatives were able to reduce the inflammatory process by 48.94% and 47%, respectively
[39].
2.4. In Silico Studies Involving Thiophene-Based Compounds with Anti-Inflammatory Properties
The search for new bioactive compounds is a long process, which takes several years, and which gradually becomes expensive, costing tens of millions of dollars before reaching the goal of having a new chemical entity capable to be used in therapy. Aiming to reduce these costs, reducing the number of molecules that need to be synthesized, reducing the number of compounds that effectively need to be tested in the various in vitro, ex vivo, and in vivo assays, several computational methods, also known as in silico methods, or CADD (Computer-Aided Drug Design) studies were developed and have been constantly improved in order to reduce cost and times of the drug design and discovery process, and increase the chances of success. In silico methods are increasingly being used in both industry and in universities. They involve understanding molecular interactions from both a qualitative and quantitative point of view-based in mathematical tools. These methods generate and manipulate three-dimensional (3D) molecular structures, calculate descriptors and dependent molecular properties (pharmacokinetic (ADME) and toxicity properties, among others), model constructions, and employ other computational drug research tools. Analysis of the molecular structure of a given system allows relevant information to be extracted, as well as predicting the potential of the bioactive compound
[40][41][42].
In silico methods are subdivided into two major general types of CADD approaches: Structure-Based Drug Design (SBDD) and Ligand-Based Drug Design (LBDD). SBDD methods are used to help investigators to predict, with precision and efficiency, in three dimensions, at a molecular level, the position (affinity) of small molecules (drug candidates or ligand) to molecular targets, usually proteins (enzymes) and nucleic acids (DNA and RNA). Where molecular docking is one of the most widespread and most used tool. In addition, LBDD approaches have been used when only the structure of the ligands are known, or when the data on the biological activities of those ligands were known. LBDD allows determinate the correlations between chemicals structures and the physicochemical properties of ligands and their biological activities. The three main categories of LBDD are: (a) pharmacophore models, which make it possible to identify the essential characteristics of the ligands for maintenance of biological activity; (b) Quantitative Structure–Activity Relationships (QSAR), which results in quantitative activity data based on the physicochemical properties of the ligands; and (c) similarity searching, which helps to predict the biological activity and the physicochemical properties of ligands based on existing data from other ligands with similar chemical structures
[40][41][42].
In this context, Sagaama and Issaoui
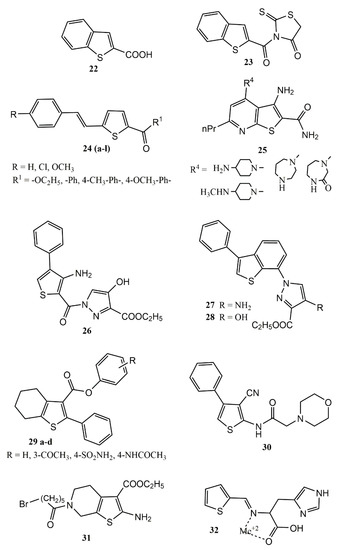
[43] performed a theoretical study involving molecular geometry, vibrational, pharmaceutical (
1H and
13C Nuclear Magnetic Resonance (NMR) and UV-vis spectrum), and electronic properties (TD-DFT (time-dependent density-functional theory), HOMO-LUMO transitions (highest occupied molecular orbital and lowest unoccupied molecular orbital)), Hirshfeld surfaces, and molecular docking, using as a prototype 1-benzothiophene-2-carboxylic acid (2BT) (22) (
Figure 5). Molecular docking was carried out using the iGEMDOCK program and Discovery studio software, against several targets, including: Human Immunodeficiency Virus type 1 (HIV) (PDB id: 1DLO), Bat SARS-like coronavirus (6LU7) (PDB id: 6LU7), and the inflammatory targets COX-2 (PDB id: 3LN1) and 5-LOX (PDB id: 3V92). The binding energy for COX-2 and 5-LOX, were respectively −81.44 and −72.48 kcal/mol.
Figure 5. Chemical structures of thiophene-based compounds with anti-inflammatory properties in in silico studies.
Molecular docking with a hybrid compound (23) containing 2BT and rhodamine (Figure 5) was performed against the COX-2 and 5-LOX enzymes, and it was revealed that the hybrid presents increased interaction energy against these inflammatory targets with respective binding energies of −98.37 and −91.07 kcal/mol. The studies suggest that (2BT) (22), and especially the hybrid 2BT+rhodamine (23), are potential competitive dual inhibitors COX-2/5-LOX, and can be used as prototypes for development of new anti-inflammatory drugs.
Karthick, Balachandran and Perumal
[44] also performed spectroscopic investigations with compound
22 (Uv-vis spectra and FT-IR (Fourier-transform infrared)), intra and intermolecular interactions, and molecular docking (
Figure 5). To evaluate anti-inflammatory potential, molecular docking studies were carried out using SWISSDOCK webserve, against COX-2 (PDB id: 1CX2) and Prostaglandin H2 synthase (PDB id: 1PTH). Although the binding affinity value were low, the authors confirmed the potential anti-inflammatory activity of compound
22, which presented binding affinity values of −6.13 kcal/mol (for COX-2), and −6.28 kcal/mol (for Prostaglandin H2 synthase).
Molecular docking was performed on COX-2 (PDB id: 3LN1) and 5-LOX (PDB id: 3V99) enzymes using the software Molecular Operating Environment, and it was observed that compound 22 (Figure 5) displayed significant dual COX-2/5-LOX inhibitory activities with respective binding affinities of −12.47 and −11.79 kcal/mol. In silico physicochemical and pharmacokinetics properties were also predicted using the Molinspiration online property calculation toolkit, MolSoft software, the PreADMET calculator, and the Osiris property explorer. Compound 22 passed well in all filters (ADMET, physicochemical, and drug-like properties) demonstrating lead compound potential for dual COX-2/5-LOX inhibitor development.
3. Final Remarks
This work demonstrates the importance of thiophene-based compounds as privileged structures in drug design and in discovery of novel anti-inflammatory agents. The vast majority of their planned and synthesized derivatives present anti-inflammatory activity superior to the reference NSAIDs as was shown in in vitro, and in silico, and in vivo assays.