1000/1000
Hot
Most Recent

MYOC encodes a secretary glycoprotein of 504 amino acids named myocilin. MYOC is the first gene to be linked to juvenile open-angle glaucoma (JOAG) and some forms of adult-onset primary open-angle glaucoma (POAG).
Glaucoma includes a group of eye disorders characterized by progressive cupping of the optic nerve head and visual field loss [1]. In fact, this optic neuropathy includes the (i) loss of neural tissue; (ii) activation of glial cells; (iii) tissue remodeling and change of intra-ocular pressure (IOP) that may lead to apoptosis of the retinal ganglion cells [1][2]. One of the mechanisms involved in apoptosis of the retinal ganglion cells includes posterior bowing of the lamina cribrosa, followed by the blockage of the axonal transport and interruption of the delivery of neurotrophins from the superior colliculus to the retinal ganglion cell body [3]. Untreated glaucoma is the leading cause of irreversible blindness in the world. A model projection revealed that, by 2020, 79.6 million of people worldwide will be affected by glaucoma and 5.9 million of individuals will be concerned by glaucoma-associated bilateral blindness [4].
Primary open-angle glaucoma (POAG), also named chronic glaucoma, is the most common form of glaucoma [4]. Unlike angle-closure glaucoma (ACG), aka acute or narrow-angle glaucoma, OAG has a wide and open angle between the iris and cornea (Figure 1). There are several risk factors associated with POAG, including demographic, familial, systemic and ocular factors [5]. Both increased age and ancestry are risk factors indentified in transversal and longitudinal studies. The presence of a family history of glaucoma, another well-established risk factor for the development of POAG, has been described among populations of different ethnicities [5]. The early-onset form of POAG, called juvenile open-angle glaucoma (JOAG), frequently shows a Mendelian pattern of inheritance, whereas the most prevalent sub-form, called adult-onset POAG, is inherited as a complex trait in the majority of the cases [6]. Systemic factors, including low perfusion pressure of the optic nerve head, have also been reported in various studies [7]. Finally, increased IOP is a strong risk factor for POAG. Population-based data have shown a positive association between IOP and the incidence of glaucoma [5].
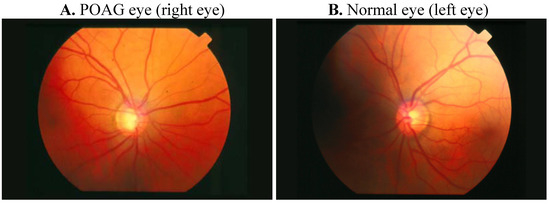
Figure 1. Comparative retinography photograph from the same patient. The optic disc is showed as a bright area where blood vessels converge.
IOP is essential to keep the shape of the eye, allowing the images to be focused accurately onto the retina, and to maintain an adequate intraocular metabolism. The aqueous humor, produced in the ciliary body, contains oxygen and nutrients to nourish the asnterior segment of the eye. It is drained from the eye into the bloodstream through the sieve-like trabecular meshwork (TM). TM is thought to regulate aqueous humor outflow to control IOP. In brief, TM is composed of cells and matrix and is represented by three structurally different regions: (i) the inner uveal meshwork; (ii) the deeper corneoscleral meshwork; (iii) the juxtacanalicular tissue (aka the cribriform meshwork), which is directly adjacent to the inner wall of Schlemm's canal [8]. In POAG eyes, there is a partial blockage within the TM, especially at the juxtacanalicular tissue, restricting the drainage of aqueous humor and increasing IOP (Figure 2). There is also an increase of the extracellular matrix, and an accumulation of banded fibrillar elements that are embedded in different glycoproteins, denominated "plaque material", leading to an increase of the trabecular outflow resistance [9].
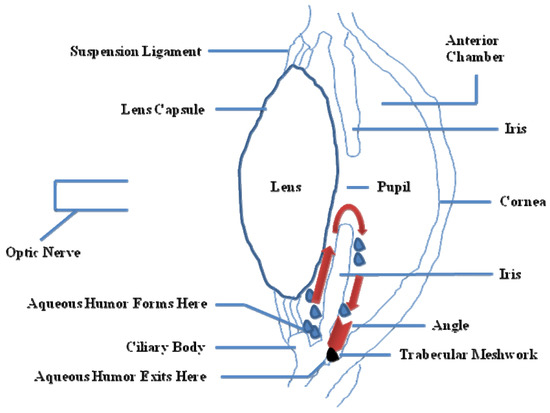
Figure 2. Partial side view of the human eye. Red arrows show the drainage of the aqueous humor from the inner chamber to the Schlemm's canal at the outer edge of the iris. In an open angle glaucoma (OAG) eye, fluid is unable to exit at the angle and stays within the eye, thus increasing the pressure (IOP).
It has been difficult to find a single marker protein to identify a human TM cell, although the pronounced phagocytosis rate shown by these cells can be used as a specific behavior [10]. One of the most important characteristic of the human TM cell is the increased expression of genes such MYOC following exposure to glucocorticoids (e.g., dexamethasone) [10][11][12], or αB Crystallin, after transforming growth factor-β (TGF-β), heat shock or oxidative stress treatment [10].
There are usually no symptoms in the initial stages of the glaucomatous disease, but blindness can be prevented if glaucoma is diagnosed and treated early enough. The aim of treatment is to lower the target IOP. Target IOP can be defined as the IOP level where further damage to the optic nerve is likely to be prevented or delayed. Target IOP varies from case to case and depends on age, baseline, untreated IOP, and glaucoma severity [13]. IOP can be lowered by reducing aqueous humor production or by increasing aqueous humor outflow [14]. Indeed, IOP lowering is possible with the use of eye drops, laser treatment directed to TM, which causes ciliary body destruction or, trabeculectomy, a surgical procedure that includes the creation of a guarded fistula to allow communication between the anterior chamber and the subconjunctival space, reducing the resistance to aqueous humor outflow.
Eventually, as suggested from epidemiological and molecular studies, it is now well established that a genetic component may contribute to OAG, and several OAG-associated genes have been identified. The first-identified and the most-studied gene is MYOC, encoding for the myocilin protein, which is highly expressed in and secreted by the human TM [12]. Dominant mutations in MYOC are more frequently observed in JOAG (10% to 30% of the cases) than in POAG (3% to 4% of patients) [15][16]. Some mutations in the MYOC gene lead to the inhibition of mutated myocilin secretion. Secretion of wild-type myocilin (wt myoc) can also be reduced or blocked in the presence of mutated myocilin (mt myoc) [17][18][19]. It has been suggested that the intracellular accumulation of myocilin aggregates is deleterious to the TM cells, resulting in the deterioration of their function and subsequent elevation of IOP [20][21]. In the present study, we review and discuss relevant literature data about the human myocilin molecule with regards to its structure, expression, interactions and potential role(s) in POAG etiology.
In 1993, Sheffield et al. performed a linkage analysis in a five generation family with JOAG using microsatellites as genetic markers. The authors described the first locus associated with OAG, denominated GLC1A, mapped in the long arm of chromosome 1 [22]. In 1997, from families affected by autosomal dominant JOAG and POAG, Stone et al. identified mutations in the MYOC gene, a TM-inducible glucocorticoid response gene (TIGR), located in the GLC1A interval on chromosome 1q23-q24 [12]. The cDNA was then independently cloned from subtracted ciliary body [23] and retinal cDNA libraries [24].
The induction of the MYOC gene expression was initially observed in cultured TM cells following treatment with glucocorticoids such as dexamethasone (DEX) [11][25]. It is well known that the long-term use of topical ophthalmic steroids results in IOP with glaucoma, known as steroid-induced glaucoma (SIG) [26]. Interestingly, the profile of MYOC up-regulation by DEX was dose- and time-dependent, very similar to the course of development of SIG [25]. Nevertheless, the association between myocilin expression and steroid-induced IOP was not evident [27].
Although MYOC gene has been found, by Northern blot, to be expressed as a 2.3 kb transcript in many human tissues (e.g., heart, stomach, thyroid, bone marrow, thymus, prostate, colon) but not in all (e.g., brain, placenta, liver, kidney, spleen, or leukocytes), its highest abundance appeared to be restricted to ocular tissues such as iris, ciliary body, optic nerve, aqueous humor and TM [24][28][29][30][31][32][33][34]. Another study revealed, also by northern blot analysis, that MYOC is variably expressed as 2.1 and 1.8 kb transcript isoforms in eye structures [35].
The study of regulatory mechanisms governing glucocorticoid-mediated MYOC induction in human TM cells, showed that (i) the promoter region between −2548 and −1541 bp, is required for DEX induction of MYOC expression; (ii) MYOC is a delayed secondary glucocorticoid-responsive gene; (iii) MYOC mRNA is intrinsically quite stable [36]. This MYOC mRNA stability can be regulated by over-expression of optineurin, another protein associated with glaucoma [37].
Genomic sequence analysis revealed that MYOC gene is composed of three exons of 604, 126, and 782 bp, respectively [38], which spans 16 kb [39]. Independently, another study confirmed the presence of these exons, and an imperfect palindromic glucocorticoid response element in the 5-prime untranslated region (5'-UTR) was identified [40]. In fact, the 5'-UTR of MYOC gene contains TATA and CAT boxes, MIR and Alu repeat sequences, binding sites for multiple hormone and cell signaling response elements, but it lacks SP1-binding sites [25]. The 3'-UTR contains three polyadenylation signal sequences [35] and not two as previously described in [25]. The encoded protein, myocilin, belongs to a family of glycosylated proteins containing a C-terminal olfactomedin-like (OLF) domain [41][42][43]. This domain was originally identified in a glycoprotein isolated from the olfactory epithelium of frogs [44]. This family includes both secreted and membrane-bound proteins with a characteristic distribution in different tissues [45][46][47][48][49][50][51][52][53].
Sequence analysis revealed that human MYOC encodes a secreted glycoprotein, myocilin, of a molecular weight of about 55 kDa represented by 504 amino acids, which displays a leucine zipper domain, 10 putative phosphorylation sites and four potential glycosylation sites [38]. Nevertheless, a variant of MYOC with a 338 bp internal deletion removing the coding sequence for the entire leucine zipper region has also been identified in human ocular tissues [35]. Furthermore, it has been observed in aqueous humor and ocular tissues that the human wtmyoc was proteolytically cleaved between arg226 and ile227, resulting in a 35 kDa fragment containing the C-terminal OLF domain and a 20 kDa fragment containing the N-terminal leucine zipper domain [54].
Interestingly, a knowledge-based consensus modeling approach [23][55] showed that myocilin is structurally characterized of three main regions (Figure 3): (i) a N-terminal myosin-like coiled-coil region including a leucine-zipper (between amino-acids 111 and 184); (ii) a flexible linker region (between amino-acids 185 and 245); (iii) a C-terminal OLF domain (between amino-acids 246 and 504). However, this described model is somehow in partial discordance with a deletion experiment study [56] revealing, a (i) coiled-coil domain located between the amino-acids 78-105; (ii) leucine zipper region between the amino-acids 114-183; (iii) C-terminal domain between the amino-acids 245-504. Functional analysis of myocilin showed that the integrity of amino-terminal coiled-coil regions and olfactomedin homology domain are essential for extracellular adhesion and secretion, the N-terminal region being also important for extracellular interactions (ECM and/or cell surface) [56].
Up-to-date, date the 3D structure of the myocilin is unknown. Indeed, cellular studies have demonstrated temperature-sensitive secretion of myocilin mutants, but difficulties in expression and purification have precluded biophysical characterization of wt myoc and disease-causing mutants in vitro [57].
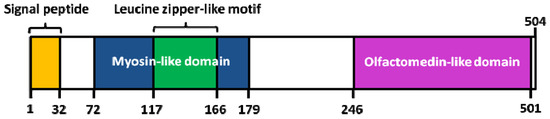
Figure 3. Structure of human myocilin. Colored areas mark the position of the signal peptide, the leucine zipper-like motif and myosin-like domain (N-terminal region), as well as the olfactomedin (OLF)-like domain (C-terminal region). Adapted from [55].
Although the MYOC gene has been studied for more than 10 years, the role of myocilin in the POAG etiology is still poorly understood [55][58]. It has been initially speculated that myocilin may cause increased IOP by reduction of the aqueous outflow [12]. Its expression in TM and ciliary body structures involved in the IOP regulation was consistent with this hypothesis [35][37].
Nevertheless, mice with targeted disruption of the MYOC gene (Myoc−/−) were: (i) viable; (ii) fertile; (iii) without any discernible phenotype; (iv) with a normal IOP, indicating that POAG is not caused by MYOC haplo-insufficiency but, might be due to a gain of function [59]. Furthermore, the over-expression of wt myoc to a level similar to that induced by glucocorticoids in the eyes of transgenic mice did not elevate IOP or display any OAG phenotype [60].
Concordantly, a patient examined with a complex deletion of the maternal copy of chromosome 1, including the entire MYOC gene, did not present elevated IOP or signs of OAG [61]. Finally, the absence of OAG phenotype in an elderly woman homozygous for the Arg46Stop mutation [62] along with the absence of glaucoma in people hemizygous for MYOC [61], suggested that the loss of functional myocilin is not critical for glaucoma development or for normal eye functioning.
Interestingly, elevated amounts of wt myoc in the aqueous humor of transgenic mice caused significant changes in expression of genes involved in cell adhesion and cell-matrix interactions, supporting a role for myocilin in modulating cellular adhesion [63].