2. Supplemental Omega-6 FA and Female Reproduction
Cow–calf systems are the foundation for global beef industries by determining the number of cattle available for harvest. Reproductive failure is a key factor limiting productivity in cow–calf operations, and pregnancy loss has been recognized as one of the main reproductive challenges in cattle
[11]. Although ≥90% of fertile beef females effectively conceive after a single service, nearly 50% remain pregnant 30 days after service and even less females give birth to a live calf
[12]. Management interventions to minimize pregnancy loss and promote embryonic survival are thus warranted, including supplementation with omega-6 FA. Linoleic acid and its omega-6 derivatives, however, serve as a precursor for prostaglandin (PG) F2α synthesis
[13], which triggers luteolysis and has embryotoxic effects during early gestation
[14]. For this reason, omega-6 FA supplementation was initially perceived as detrimental to the reproductive performance of beef cows
[5].
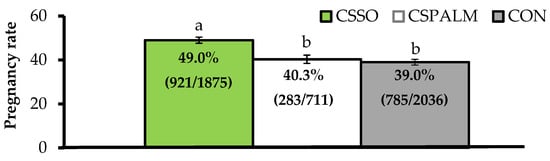
Differing from this latter concept, our research group reported that supplementing omega-6 FA via CSSO to beef cows after artificial insemination (AI) increased pregnancy rates by 25%
[15][16]. Across a series of trials, grazing
Bos indicus beef cows supplemented with 100 g/day of CSSO for 28 days beginning after AI had greater pregnancy rates compared with cows supplemented with 100 g/day of Ca soaps of palm oil (iso-caloric and iso-lipidic control rich in palmitic acid) or unsupplemented (CON) cows (). These results provide evidence that omega-6 FA supplementation improved, and did not impair
[5], the reproductive performance of beef females. Moreover, increased pregnancy rates resulting from omega-6 FA were associated with pregnancy establishment because CSSO was offered during the early embryonic period
[17], and independent of their contribution to energy intake as CSPALM resulted in similar pregnancy rates to CON.
Figure 1. Pregnancy rates (as %) after artificial insemination in beef cows receiving Ca soaps of soybean oil (CSSO), Ca soaps of palm oil (CSPALM), or CON (unsupplemented) for 28 days after AI
[15][16]. All values reported are least square means ± standard error (represented as error bars). Means with different superscripts differ (
p ≤ 0.05). Information within parentheses indicate number of pregnant cows/total cows inseminated.
To provide biological support of the findings from Lopes et al.
[15][16], Cooke et al.
[18] investigated FA incorporation into reproductive tissues and physiological responses associated with pregnancy establishment. Grazing
B. indicus beef cows were supplemented or not (CON) with 100 g/day of CSSO and slaughtered 19 days after AI. Cows receiving CSSO had greater incorporation of linoleic acid and its omega-6 derivatives into plasma, endometrium, corpus luteum, and conceptus. More specifically, CSSO supplementation increased intake and intestinal absorption of linoleic acid, which in turn was incorporated, elongated, desaturated, and accumulated into reproductive tissues, including as arachidonic acid in the conceptus (). These authors also evaluated factors associated with embryonic development and early pregnancy establishment on day 19 of gestation. These included conceptus size, mRNA expression of genes associated with pregnancy development in endometrial and luteal samples, and mRNA expression of interferon-tau (IFN-τ) by the conceptus; the conceptus-derived signal for maternal recognitions of pregnancy
[17]. The increase in omega-6 FA accumulation, however, did not impact any of these variables, despite a tendency for increased IFN-τ concentration in uterine flushes collected from CSSO cows (10.9 vs. 7.3 ng/mL). Cows were slaughtered 19 days after AI to recover elongated conceptuses that still expressed IFN-τ mRNA
[19] and provided enough tissue for both FA and mRNA expression analyses. The physiological processes responsible for pregnancy signaling to maternal tissues occur near days 15 to 17 of gestation
[17]. Hence, Cooke et al.
[18] evaluated maternal tissues and conceptuses after the critical period for pregnancy recognition, which prevented proper assessment of how omega-6 FA impacted expression of genes that mediate pregnancy establishment.
Table 1. Concentrations of fatty acids (FA) in samples collected on day 19 of gestation from
B. indicus beef cows receiving or not (CON; n = 9) Ca soaps of soybean oil (CSSO; n = 9) after artificial insemination. Values reported are least square means ± standard error. Adapted from Cooke et al.
[18].
| Item |
CON |
CSSO |
p = |
| Plasma (mg of FA/g of plasma) |
|
|
|
| Linoleic acid |
0.275 ± 0.022 |
0.540 ± 0.021 |
<0.01 |
| Arachidonic |
0.023 ± 0.005 |
0.025 ± 0.004 |
0.74 |
| Total omega-6 FA |
0.296 ± 0.023 |
0.565 ± 0.022 |
<0.01 |
| Endometrium (mg of FA/g of tissue) |
|
|
|
| Linoleic acid |
0.144 ± 0.043 |
0.358 ± 0.044 |
<0.01 |
| Arachidonic |
0.241 ± 0.061 |
0.266 ± 0.061 |
0.77 |
| Total omega-6 FA |
0.549 ± 0.136 |
0.938 ± 0.136 |
0.05 |
| Corpus luteum (mg of FA/g of tissue) |
|
|
|
| Linoleic acid |
3.935 ± 0.543 |
7.035 ± 0.543 |
<0.01 |
| Arachidonic |
4.731 ± 0.349 |
4.942 ± 0.350 |
0.67 |
| Total omega-6 FA |
12.72 ± 1.19 |
17.78 ± 1.19 |
<0.01 |
| Conceptus (mg of FA/g of tissue) |
|
|
|
| Linoleic acid |
0.022 ± 0.059 |
0.174 ± 0.062 |
0.08 |
| Arachidonic |
0.086 ± 0.043 |
0.312 ± 0.043 |
<0.01 |
| Total omega-6 FA |
0.384 ± 0.753 |
2.045 ± 0.755 |
0.13 |
To complement the results from Cooke et al.
[18], Cipriano et al.
[20] focused on conceptus- and endometrial-derived responses that mediate pregnancy signaling to maternal tissues on day 15 of gestation. Grazing
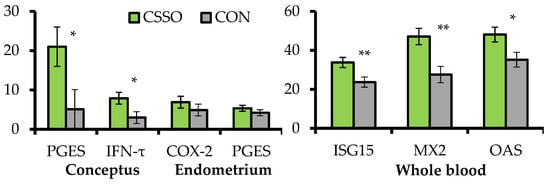
B. indicus cows were supplemented or not (CON) with 100 g/day of CSSO beginning after AI. A subset of these cows were assigned to conceptus collection via transcervical flushing with saline followed by endometrial biopsy in the uterine horn ipsilateral to the corpus luteum 15 days after AI. The remaining cows were sampled for whole blood RNA extraction 20 days after AI, and pregnancy status was verified 28 days after AI. Supplementing omega-6 FA via CSSO increased conceptus length (2.58 vs. 1.15 cm) and mRNA expression of prostaglandin E synthase and IFN-τ by the conceptus, as well as mRNA expression of interferon-stimulated genes (ISG) in the whole blood (). These results suggest that omega-6 FA supplementation enhanced conceptus development and IFN-τ synthesis during the pregnancy recognition period
[17], corroborating the increased pregnancy rates to AI when CSSO was supplemented during early gestation
[15][16]. The mRNA expression of ISGs have been used to gauge IFN-τ production and conceptus development from days 15 to 22 of gestation
[21], given that IFN-τ synthesis upregulates mRNA expression of ISGs in circulating blood leukocytes
[22]. Increased conceptus length and IFN-τ mRNA expression from supplemental omega-6 FA was associated with accumulation of arachidonic acid
[18] and upregulation of prostaglandin E synthase mRNA in the conceptus. This enzyme converts PGH
2 to PGE
2 [23], which coordinates with IFN-τ endometrial functions that are critical for conceptus development and pregnancy signaling to maternal tissues
[24]. In turn, CSSO supplementation did not impact the endometrial mRNA expression of
prostaglandin E synthase and
cyclooxygenase-2 (), suggesting that the effects of omega-6 FA on PG-related responses on day 15 of gestation may be specific to the conceptus due to heightened accumulation of arachidonic acid in this tissue and not in the endometrium
[18].
Figure 2. Expression of mRNA (relative fold change) in genes associated with pregnancy establishment in conceptus and endometrial samples collected on day 15 of gestation, and whole blood collected on day 20 of gestation from
B. indicus cows receiving or not (CON; n = 10) Ca soaps of soybean oil (CSSO; n = 10) after artificial insemination. PGES =
prostaglandin E synthase;
IFN-τ = interferon-tau; COX-2 = cyclooxygenase-2;
ISG15 = interferon-stimulated gene 15;
MX2 = myxovirus resistance 2;
OAS =
20,
50-
oligoadenylate synthetase. All values reported are least square means ± standard error (represented as error bars). Within variable, **
p < 0.01 and *
p ≤ 0.05. Adapted from Cipriano et al.
[20].
Our initial efforts in characterizing the benefits of omega-6 FA to cattle reproduction were conducted with
B. indicus cows reared in tropical conditions
[15][16][18][20]. Pregnancy establishment and overall reproductive physiology differ between
B. indicus and
B. taurus females
[25], and FA composition differs between tropical and temperate feed ingredients. Hence, Brandão et al.
[26] conducted two trials evaluating omega-6 FA supplementation via CSSO to
B. taurus cows in temperate conditions. In the first trial, grazing Angus cows were supplemented with 100 g/day of CSSO or prilled saturated fat (iso-caloric and iso-lipidic control; CON+) for 21 days after AI. Similar to the findings from Lopes et al.
[15][16], pregnancy rates following AI were increased by 17% in cows supplemented with omega-6 FA (). The companion trial focused on conceptus- and endometrial-derived responses that mediate pregnancy signaling to maternal tissues with a design similar to Cipriano et al.
[20], using Angus × Hereford cows that received 100 g/day of CSSO or CON+ beginning after AI. Supplementing omega-6 FA upregulated mRNA expression of IFN-τ by the conceptus and ISG in the whole blood, but did not increase conceptus length (11.3 vs. 11.4 cm for CSSO and CON, respectively) and mRNA expression of
prostaglandin E synthase. Conceptus length across treatments was 11.4 ± 1.9 cm in Brandão et al.
[26] and 2.4 ± 0.5 cm in Cipriano et al.
[20], suggesting that
B. taurus conceptus may be at an advanced stage of elongation on day 15 of gestation compared with
B. indicus conceptus, and past the stage in which omega-6 FA impacts conceptus growth and expression of
prostaglandin E synthase. Nevertheless, results from Brandão et al.
[26] confirmed that omega-6 FA supplementation via CSSO to
B. taurus cows also upregulated IFN-τ synthesis by the conceptus during the pregnancy recognition period, leading to increased pregnancy rates following fixed-time AI.
Table 2. Pregnancy rates and expression of mRNA (relative fold change) of genes associated with pregnancy establishment in
B. taurus cows receiving Ca soaps of soybean oil (CSSO) or prilled saturated fat (CON+) after artificial insemination. Values reported are least square means ± standard error. Adapted from Brandão et al.
[26] 1.
| Item |
CON+ |
CSSO |
p = |
| Pregnancy rates to AI (n = 11/treatment), % |
51.7 ± 4.1 |
60.2 ± 4.2 |
0.01 |
| Physiological responses (n = 9/treatment) |
|
|
|
| Endometrium, mRNA expression |
|
|
|
| Cyclooxygenase-2 |
5.11 ± 1.32 |
4.88 ± 1.33 |
0.89 |
| Prostaglandin E synthase |
7.40 ± 1.05 |
5.76 ± 1.15 |
0.30 |
| Conceptus, mRNA expression |
|
|
|
| Interferon-tau |
12.1 ± 3.6 |
21.3 ± 3.4 |
0.05 |
| Prostaglandin E synthase |
2.50 ± 0.49 |
2.22 ± 0.48 |
0.69 |
| Whole blood, mRNA expression |
|
|
|
| Interferon-stimulated gene 15 |
29.8 ± 4.9 |
43.1 ± 4.3 |
0.04 |
| Myxovirus resistance 2 |
20.1 ± 2.8 |
20.2 ± 2.5 |
0.98 |
| 20,50-oligoadenylate synthetase |
18.3 ± 2.9 |
26.8 ± 2.6 |
0.03 |
Collectively, supplementing omega-6 FA via CSSO increased incorporation of these FA into maternal and embryonic tissues and promoted IFN-τ synthesis by the conceptus during the maternal pregnancy recognition period, leading to increased pregnancy success in beef cows. These outcomes were generated across several research trials using nearly 6000 beef cows from different subspecies and managed in different environments, and were independent of the energy contribution of omega-6 FA given that iso-caloric and iso-lipidic control supplements were included. Hence, omega-6 FA supplementation is a nutritional alternative to enhance the reproductive efficiency of B. taurus and B. indicus beef cows reared in temperate and tropical environments.
3. Supplemental Omega-6 FA and Developmental Programming
The embryonic, fetal, and neonatal periods are the stages of life in which most developmental processes occur
[27]. Nutrient supply during these periods exerts long-term consequences on the growth, development, and metabolic functioning of the offspring
[28], leading to the concept of developmental programming
[29]. Fetal developmental is sensitive to maternal nutrient status from oocyte maturation to parturition
[30][31], and developmental plasticity remains susceptible to environmental stimuli during early postnatal life
[32]. Dietary FAs provide a specific opportunity to nutritionally modulate developmental programming, as they differentially regulate expression of genes across metabolic tissues. For example, omega-3 FA limits adipose tissue accumulation by suppressing adipocyte differentiation
[33][34], whereas omega-6 FA has been described as proadipogenic
[35][36]. The fetal stage is critical for skeletal muscle and intramuscular adipocyte development
[37][38]; hence, omega-6 FA supplementation during gestation can potentially enhance adipogenesis and thereby sites for marbling formation later in life
[39].
4. Supplemental Omega-6 FA to Growing and Finishing Cattle
Weaning and feedlot receiving are two of the most stressful events in the beef production cycle, when cattle are exposed to a variety of physiological and physical stressors, including road transport, exposure to novel diets and environments, and comingling with new animals
[40]. The combination of all of the stressors stimulates neuroendocrine and inflammatory reactions that directly impair cattle immunocompetence and productivity, leading to BRD incidence and reduced performance upon feedlot arrival
[41]. Hence, strategies to increase the immunocompetence of cattle during the initial phases of the feedlot are warranted, including the use of omega-6 FA based on its immunomodulatory properties
[42]. Research from our group demonstrated that omega-6 FA supplementation via CSSO to cattle upon feedlot arrival decreased plasma concentrations of inflammatory markers, but reduced feed intake and subsequent cattle ADG
[8]. For this reason, our group evaluated omega-6 FA supplementation prior to feedlot arrival, by supplementing CSSO during a post-weaning preconditioning program
[9]. Steers supplemented with omega-6 FA via CSSO during preconditioning had a greater feedlot-received ADG, which was attributed to reduced plasma concentrations of proinflammatory cytokines (). Moreover, CSSO steers had improved carcass marbling upon slaughter, which was associated with greater ADG upon feedlot arrival and potentially with metabolic imprinting effects, as omega-6 FA was supplemented when steers were 6 months old
[9]. Hence, omega-6 FA supplementation prior to feedlot arrival should also be considered as a nutritional intervention to improve initial health and performance of feedlot cattle.
Table 3. Performance and health responses from steers supplemented or not (CON; n = 6) with Ca soaps of soybean oil (CSSO; n = 6) for 28 days prior to feedlot arrival (day 0). Values reported are least square means ± standard error. Adapted from Cooke et al.
[9].
| Item |
CON |
CSSO |
p = |
| Plasma tumor necrosis alpha, pg/mL (log) |
|
|
|
| Day 0 (arrival) |
1.74 ± 0.21 |
1.91 ± 0.21 |
0.58 |
| Day 1 |
1.88 ± 0.23 |
2.00 ± 0.22 |
0.67 |
| Day 3 |
2.23 ± 0.20 |
1.55 ± 0.20 |
0.03 |
| Feedlot average daily gain, kg/d |
|
|
|
| Initial phase (day 1 to 144) |
1.17 ± 0.02 |
1.25 ± 0.02 |
0.02 |
| Final phase (day 145 to slaughter) |
2.10 ± 0.05 |
2.09 ± 0.05 |
0.86 |
| Carcass traits |
|
|
|
| Hot carcass weight, kg |
394 ± 6 |
402 ± 6 |
0.31 |
| Longissiumus muscle area, cm2 |
94.7 ± 1.5 |
92.0 ± 1.6 |
0.23 |
| Yield grade |
3.16 ± 0.10 |
3.48 ± 0.11 |
0.04 |
| Marbling |
444 ± 18 |
515 ± 19 |
0.01 |
| Backfat, cm |
1.55 ± 0.06 |
1.63 ± 0.06 |
0.38 |
Beef cattle are typically backgrounded on pasture after weaning in areas where forage is available for grazing
[43], although supplemental nutrients are often required in this practice to meet the requirements of growing cattle
[44]. Hess et al.
[5] reviewed multiple studies in which omega-6 FA was supplemented to grazing cattle, but using grains and oilseeds highly susceptible to ruminal biohydrogenation
[10]. To fill this gap in knowledge, Cappellozza et al.
[45] evaluated performance and nutrient intake of grazing
B. indicus bulls supplemented with omega-6 FA via CSSO. In this study, ADG was increased in bulls offered a grain-based supplement at 0.3% of their body weight fortified with omega-6 FA compared with bulls receiving an iso-caloric and iso-nitrogenous control supplement (0.92 vs. 0.81 kg/day, respectively). These authors also noted that bulls supplemented with omega-6 FA consumed less water (4.11 vs. 4.96% of body weight), and hypothesized that this outcome was due to reduced ruminal caloric increment from inclusion of CSSO into the supplement
[45]. More specifically, CSSO partially replaced corn to maintain the supplement’s iso-caloric and iso-nitrogenous status, whereas ruminal fermentation of starch resulted in greater heat production compared with rumen-inert fats
[45][46].
Another area of limited research is the inclusion of omega-6 FA into feedlot diets, as these FA from natural sources can disrupt ruminal function, feed intake and efficiency, and overall cattle performance
[5]. The use of CSSO may partially alleviate these concerns, as supplementing Ca soaps of cottonseed oil improved feed efficiency of feedlot
B. indicus bulls compared with cohorts receiving isocaloric and isonitrogenous diets
[47]. Accordingly, Nascimento et al.
[47] investigated the inclusion of omega-6 FA via CSSO, or a mixture of palm, soybean, and cottonseed oils fed as Ca soaps into feedlot diets (CSMIX). Supplemented CSSO or CSMIX increased energy intake, feed efficiency, ADG, and carcass merit of
B. indicus finishing bulls compared with cohorts not receiving supplemental fat (). In turn, cattle performance and carcass traits were not improved by omega-6 FA supplementation via CSSO compared with the saturated + monounsaturated FA provided by the CSMIX (). Therefore, omega-6 FA inclusion via CSSO to feedlot diets improved cattle performance and efficiency by increasing the energy density of the diet, whereas a combination of saturated + monounsaturated FA appears to be more favorable for feedlot productivity and carcass quality
[48][49].
Table 4. Performance and carcass traits of feedlot bulls supplemented or not (CON; n = 16) with Ca soaps of soybean oil (CSSO; n = 16) or a mixture of palm, soybean, and cottonseed oils (CSMIX; n = 15) until slaughter. Values reported are least square means ± standard error. Adapted from Nascimento et al.
[47] 1.
| Item |
CON |
CSSO |
CSMIX |
C1 |
C2 |
| Performance |
|
|
|
|
|
| Average daily gain, kg/d |
1.14 ± 0.04 |
1.37 ± 0.05 |
1.48 ± 0.05 |
<0.01 |
0.11 |
| Feed efficiency, g/kg |
156 ± 3 |
168 ± 3 |
183 ± 3 |
<0.01 |
<0.01 |
| Final body weight, kg |
476 ± 6 |
508 ± 7 |
524 ± 7 |
<0.01 |
0.13 |
| Carcass traits |
|
|
|
|
|
| Hot carcass weight, kg |
268 ± 4 |
284 ± 4 |
297 ± 4 |
<0.01 |
0.03 |
| Longissiumus muscle area, cm2 |
67.8 ± 1.88 |
70.4 ± 1.94 |
75.4 ± 1.94 |
0.04 |
0.08 |
| Backfat, cm |
0.318 ± 0.035 |
0.439 ± 0.039 |
0.448 ± 0.040 |
0.01 |
0.87 |
5. Conclusions
This review compiled recent research on omega-6 FA supplementation via CSSO to beef cattle, and its benefits to production efficiency across different environments and sectors of the beef industry. Supplementing omega-6 FA increased the reproductive efficiency of beef cows by promoting the processes associated with early pregnancy establishment. Omega-6 FA also elicited positive effects during periods of developmental plasticity, such as gestation and early postnatal life. Supplementing omega-6 FA to beef cows during late gestation resulted in alterations in tissue differentiation and improved health and productivity of offspring. Similar effects on developmental programming were noted when omega-6 FA was supplemented to young calves. Lastly, supplementing omega-6 FA to growing cattle receiving forage-based diets resulted in enhanced immunocompetence, growth, and carcass merit, although such benefits were not evident when omega-6 FA was provided to feedlot cattle consuming high-concentrate diets. Collectively, this review provides research-based evidence that omega-6 FA supplementation via CSSO is a sustainable approach to improve beef production efficiency.