1000/1000
Hot
Most Recent

Surface functionalization with carbon nanomaterials in dental and orthopedic implants has emerged as a novel strategy for reinforcement and as a bioactive cue due to their potential for osseointegration.
To date, metal-based dental and orthopedic implant materials, including titanium (Ti), stainless steel, and cobalt–chromium (CoCr), have been widely used because of their suitable properties, such as high mechanical strength, light-weight chemical stability, and nonimmunogenic property. For successful implantation of orthopedic and dental implants, biometric stability immediately after implant insertion is one of the most important steps. Despite the wide clinical utilization of Ti implants, there are still potential risks because of the inherent bioinert and easily oxidizable characteristics. For example, the oxide layer of the surface of Ti often leads to thrombosis between the surface and surrounding tissue, which creates an oral cavity that promotes microbial reproduction [1][2]. Moreover, during the operation, inflammation around the surgical sites may occur due to external heat or pressure. This hinders the normal growth of new bone around the surgical sites and results in weak bonding between the bone and implant [3][4][5]. The risk of implantation failure rises particularly rapidly in the elderly, who have decreased bone mass and a degraded microstructure of bone tissue caused by senile disorders, such as diabetes and osteoporosis, resulting in fragile bone tissue. Recent studies have focused on surface functionalization by endowing implants’ biofunctionalities for the reduction of surgery failure. The above problems can be solved by improving the surface properties of implant materials. Therefore, various surface functionalization methods have been extensively employed to enhance the biofunctionality of implants (Figure 1).
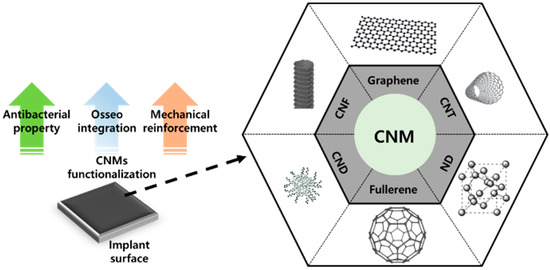
Figure 1. Surface functionalization with carbon nanomaterials (CNMs) containing carbon nanofibers (CNFs), graphene, carbon nanotubes (CNTs), nanocrystalline diamond (ND), carbon nanodots (CNDs), and fullerene.
Surface functionalization is a powerful tool for the alteration of physicochemical properties of implant surface that allows preferred bioactivity and reduced adverse effects to be achieved. Growth factors and inducers have been administered to promote osteogenesis; however, they are complicated, expensive to produce, and easily degraded in vivo [6]. Significant advances have been made in surface functionalization by adopting a vast area of materials that endow substrates with specific characteristics (e.g., polymers and inorganics) [7][8]. Nanomaterial (NM)-based coatings in particular offer several advantages: (a) tunable micron/nanometer-sized multiporous topography, (b) high specific surface area, (c) unique cell–matrix interaction, and (d) mechanical reinforcement. These regulate bone cell behaviors and improve mechanical properties. Furthermore, it is important to pursue stable and long-lasting coating layers to confer bioactive (i.e., osteoconductive and osteogenic) properties in vivo. Therefore, many novel strategies have been introduced to achieve robust and stable coatings as described herein.
Carbon nanomaterials (CNMs) are some of the most important members of the NM family. The discovery and emergence of CNMs have impacted many aspects of nanotechnology and have contributed to significant developments in physics, electronics, optics, mechanics, biology, and medicine. Many CNMs have gained increasing attention in the biomedical field due to their extraordinary characteristics. For example, fullerenes and carbon nanotubes (CNTs) have been widely studied for numerous therapeutic and pharmaceutical purposes [9][10][11]. Moreover, other CNMs like graphene and nanocrystalline diamond (ND) have become popular in the past decade due to the maturation of various fabrication and modification techniques [12][13][14]. CNMs in particular have been shown to be capable of facilitating cellular behaviors such as adhesion; migration; proliferation; and differentiation into several lineages, including myogenesis, neuritogenesis, and osteogenesis [15][16][17][18][19][20]. Therefore, various CNMs, such as graphene, CNTs, ND, carbon nanofibers (CNFs), fullerene, and carbon nanodots (CNDs), have been considered to possess excellent potential for surface functionalization materials of implants due to their osteogenesis-inducing property and mechanical reinforcement property.
The main idea of physicomechanical modification is to induce the physical adsorption of CNMs on implant surfaces by plasma spraying, gas or vapor radiation, solution treatment, or desorption, or by using mechanical methods such as roughening and micromanipulation. Most of the physical modification methods feature advantages such as a short processing time, simple equipment, and no preference for the intrinsic properties of the implant material. However, several disadvantages exist, including inhomogeneity, weak bonding and wear resistance, and difficulty to coat the inner surface of small holes.
Hydroxyapatite (HAp) is used as a coating material for Ti implants due to its osteoinduction and biocompatible pCSroperty [21][22]. However, neat HAp is mechanically disadvantageous as it exhibits poor wear resistance and fracture toughness [23]. Therefore, it is not possible to solve post-transplant side effects such as arthroplasty prostheses loosening with HAp-coated implants [24][25][26]. However, CNMs’ great potential for mechanical reinforcement of brittle HAp presents a way to overcome this issue. CNT has been extensively applied as reinforcement to enhance weak mechanical characteristics of ceramics such as HAp and Al2O3 as well as to facilitate osteoinduction [27]. Plasma spraying is a physical vapor deposition technique that uses high-velocity spraying of molten powder onto an implant surface [28]. Plasma spraying is the only US Food and Drug Administration (FDA)-approved implant coating method and forms a dense and adherent coating on implant surfaces [29]. Balani et al. and Lahini et al. proved that plasma spraying of CNT-HAp on the surface of Ti improves fracture toughness and wear resistance [30][31]. Facca et al. plasma sprayed CNT-reinforced HAp on the surface of titanium and confirmed enhanced mechanical and osteoinduction properties (Figure 2) [32]. Because there is a limited number of reports exploring in vivo responses of HAp-CNT-coated implants, this study focused on the in vivo response of implants embedded in rats and mice. The results indicated that incorporated CNT did not induce adverse or cytotoxic events, and normal bone growth was observed around the HAp-CNT-coated implant. Interestingly, the addition of CNT significantly increased new bone formation when compared to bare HAp-coated implants. The elastic modulus of the newly formed bone was similar to that of the distant bone, suggesting excellent integrity of the implant and bone. Similarly, Xie et al. plasma coated graphene-reinforced calcium silicate (CS) on a Ti implant and evaluated coating quality and in vivo osseointegration [33]. The graphene and CS were evenly coated on the surface of Ti, and the incorporated graphene enhanced the wetting behavior and wear properties via the formation of interfacial bonding between the CS particles. Moreover, in vivo experiments indicated that graphene did not hinder the biocompatibility and significantly increased the bone–implant contact ratio after three months of implantation when compared to the bare CS coating. On the other hand, ultrasonic atomic spraying is a physical coating method that enables thin coating of high performance and quality with accurate control of process parameters [34]. Li et al. fabricated a graphene oxide (GO)-coated Ti implant and evaluated the feasibility of coating quality and osteogenesis-inducing ability [35]. Ultrasonic atomic spraying retained the original structure of GO and deposited a thin and uniform layer on Ti substrate. Osteogenic differentiation of seeded bone marrow-derived mesenchymal stem cells (BM-MSCs) was characterized by upregulated osteogenic markers containing alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OPN). The paper analyzed the mechanism of osteogenesis of BM-MSCs by Western blotting of cytoskeletal proteins. GO stimulated expression of the focal adhesion kinase (FAK) and mitogen-activated protein kinase (MARK) signaling pathways related to extracellular-signal-regulated kinase (ERK)1/2, P38, and c-Jun N-terminal kinase (JNK), and several osteogenic markers were then upregulated in a cascade. This suggests that the formation of focal adhesion between stem cells and GO induces spontaneous osteogenic differentiation, which leads to increased osseointegration in vivo.
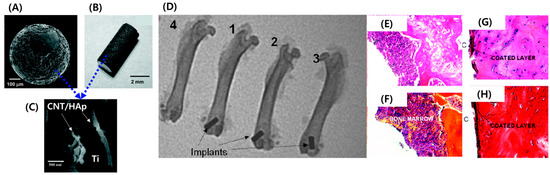
Figure 2. HAp/CNT-coated implant showing (A) a spherical Ti bead, (B) a Ti road, and (C) the top surface of the plasma-sprayed HAp-CNT coating with embedded CNTs in HAp. (D) X-ray images of rat femoral bones after one-month implantation showing (1) an uncoated Ti implant, (2) an HAp-coated Ti implant, (3) an HA-CNT-coated implant, and (4) no implant. (E–H) Histological results (40×) for mice bones that were implanted with an HA-CNT-coated implant fabricated as (E,F) spherical beads and (G,H) a rod-shaped implant. (E,G) Mallory coloration images and (F,H) hematoxylin and eosin coloration images. Copyright © 2011 American Chemical Society, Reference [32].