Artificial sweeteners have gained increasing attention as dietary assessment tools to help combat the obesity epidemic by providing a sweet taste without the extra calories. Individuals widely use non-nutritive sweeteners (NNS) in attempts to lower their overall daily caloric intake, lose weight, and sustain a healthy diet. Recent studies have suggested that NNS consumption can induce gut microbiota dysbiosis and promote glucose intolerance in healthy individuals that may result in the development of type 2 diabetes mellitus (T2DM).
1. Introduction
Artificial sweeteners have gained increasing attention as dietary assessment tools to help combat the obesity epidemic by providing a sweet taste without the extra calories
[1]. Taste has a significant role in human perception of food quality, contributing to its overall pleasure and enjoyment. To this end, the development of sweeteners as food additives that mimic the sweet taste of natural sugars suggest promise
[2]. These artificial sweeteners are classified as nutritive or nonnutritive, both of which enhance the flavor and texture of food. Nutritive sweeteners contain carbohydrates and provide calories (energy). Non-nutritive sweeteners (NNS) are very low calorie or zero calorie alternatives that provide minimal or no carbohydrates or energy
[3].
As part of dietary intake, NNS consumption can modulate energy balance, and metabolic functions through several peripheral and central mechanisms, suggesting that NNS are not inert compounds as once thought
[4]. However, the specific mechanism(s) and details of the effects of NNS consumption on host metabolism and energy homeostasis remain to be elucidated. This is particularly relevant as NNS have been an option for individuals to improve their health; yet, NNS consumption has been associated with increased risk factors for metabolic syndrome
[5]. Here, metabolic syndrome refers to the collection of physiological, biochemical, clinical, and metabolic factors that contribute to the increased risk of cardiovascular disease and type 2 diabetes melitus (T2DM)
[6]. Based on measurements and laboratory tests, metabolic syndrome can also contribute to hypertension, glucose intolerance, proinflammatory state, atherogenic dyslipidemia, prothrombic state
[7], and kidney disease
[8]. It is noteworthy that the cause of these health-related issues may be due to emerging contaminants in the environment worldwide and their associated risks to human health and the environment
[9]. Interestingly, one study identified a total of 24 non-nutritive artificial sweeteners studies to their occurrence in the environment from 38 locations globally across Europe, including the United Kingdom, Canada, United States, and Asia. Overall, the findings of the study indicated that non-nutritive artificial sweeteners are present in surface water, tap water, groundwater, seawater, lakes, and atmosphere
[9]. Furthermore, in a Norwegian pregnancy cohort study, sucrose-sweetened soft beverages were reported to increase the risk of congenital heart defects (CHDs) in offspring, while fruit juices, cordial beverages, and artificial sweeteners had no associations with CHD
[10].
2. Current Status on the Use of Non-Nutritive Sweeteners
Currently, the Food and Drug Administration (FDA) has approved the use of acesulfame-potassium (Ace-K), aspartame, neotame, saccharin, sucralose, and stevia (
https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm). Saccharin was discovered as early as 1876 and was the “original” artificial sweetener used in the food industry. Unfortunately, saccharin and many of its sweet alternatives have been considered to be health hazards, and as a result, are banned in many countries. Recently, other sweeteners have been developed and implemented within the food industry. In general, there are three primary types of sweeteners used in the food industry today: high-intensity sweeteners (e.g., acesulfame potassium, advantame, aspartame, neotame, saccharin, and sucralose), sugar alcohols (e.g., erythritol, glycerol, mannitol, sorbitol, and xylitol), and natural sweeteners (e.g., honey, lucuma powder, maple syrup, monk fruit known as
Siraitia grosvenorii swingle fruit extract, stevia, and yacon syrup)
[11]. These sweeteners and their uses in the food industry are summarized in
Table 1. The high-intensity sweeteners can be synthetic or natural and are classified into two categories: nutritive and non-nutritive. The majority of high-intensity sweeteners used today fall into the non-nutritive category, with the exception of aspartame. Sugar alcohols are found naturally in small amounts in fruits and vegetables but are produced commercially in larger quantities.
Table 1. Classification of Food and Drug Administration (FDA)-approved sweeteners.
|
Name
|
Brand Names
|
Applications in Food Industry
|
Relative Sweetness (Measured to Sucrose)
|
|
High-intensity Sweeteners
|
|
Saccharin
|
Sweet and Low®, Sweet Twin®, Sweet’N Low®, Necta Sweet®
|
Beverages, bases, and mixes for many food products, table sugar substitute
|
200–700×
|
|
Aspartame *
|
Nutrasweet®, Equal®, Sugar Twin®
|
Soft drinks, chewing gum, pudding, cereals, instant coffee
Also distributed as a “General Purpose Sweetener”
|
200×
|
|
Acesulfame-potassium (Ace-K)
|
Sunett®, Sweet One®
|
Beverages, candy, frozen desserts, baked goods
Heat stable so it can be used in baking
|
200×
|
|
Sucralose
|
Splenda®
|
|
600×
|
|
Neotame
|
Newtame®
|
Beverages, candy gum
|
7000–13,000×
|
|
Advantame
|
N/A
|
Baked goods, beverages, frozen desserts, frosting, chewing gum, candy, pudding, jelly and jam, gelatin
|
20,000×
|
|
Sugar Alcohols
|
|
Erythritol
|
|
Fondant, ice cream, gum, tabletop sweeteners, chocolate, dairy products, jelly, beverages
|
0.60×–0.70×
|
|
Glycerol
|
|
Dairy products, processed fruits, energy bars, jam, fondant
Often used as a thickening agent and to provide texture to food
|
|
|
Mannitol
|
|
Infant formula, frozen fish, precooked pasta, butter, chocolate flavored coatings
|
0.50×–0.70×
|
|
Sorbitol (Glucitol) *
|
|
Used as emulsifier
|
0.66×
|
|
Xylitol
|
|
Hard candy, chewing gum, mints, ice cream, chocolate, cookies, beverages, table sugar substitute
|
1×
|
|
Natural Sweeteners
|
|
Steviol glycosides
|
Natural constituents of leaves of Stevia rebaudiana (Bertoni) plant, commonly known as Stevia
|
Beverages, chewing gum, candy
|
200–400×
|
|
Luo Han Guo Monk fruit extracts
|
Siraitia grosvenorii Swingle fruit extract (SGFE)
|
Tea
|
100–250×
|
|
Lucuma powder
|
|
Beverages, pudding, granola, pastry, baked goods
|
|
* Nutritive sweetener. Content taken in part from the FDA approval of artificial sweeteners. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm and Shwide-Slavin et al. [11].
3. Future of Artificial Sweeteners in the Food Industry
There are now growing concerns over obesity and other health issues, and as a result, there will be a demand for sweet alternatives. Consumers can be classified broadly into two categories:
-
Those that are interested in having low-sugar, low-calorie options to promote a healthy lifestyle and to avoid some of the health issues associated with consuming high amounts of sugar, such as obesity, diabetes, and heart disease.
-
Those who already have with one or more of these health issues and are looking for ways to improve their diet and manage their health.
While the demand for artificial sweetener options in the beverage industry has been high, the demand for low-calorie sweeteners in place of sugar in baked goods, candies, and ice cream is increasing
[12]. This high consumer pool opens a larger market for food manufacturers, making it increasingly important to understand artificial sweeteners and the roles they play in the lives of consumers worldwide. The preferences for specific sweeteners may impact food and beverage sales, so it is important that manufacturers stay abreast of the scientific developments surrounding each sweetener and what their impact may have on the demand for that specific sweetener.
Despite FDA approval of several sucrose alternatives marked as Generally Recognized As Safe (GRAS), there remains growing concern about the potentially harmful side effects associated with NNS consumption. Although several epidemiologic studies are focusing on artificial sweetener use and weight gain, it is critical that when interpreting such studies we consider factors that affect causality, and control for confounding factors such as age, diet, and environment, as well as additional stressors that may modify microbiota composition
[13]. The gaps in our knowledge regarding how NNS consumption is implicated in host metabolism reinforces the importance of research needed to understand the mechanistic action of NNS on the body.
4. Physiological Effects of Non-Nutritive Sweeteners
Increased incidence of obesity and diabetes make NNS and their low caloric value even more favorable diet supplements. It is generally accepted that high sugar diets contribute to metabolic disorders
[14]. The National Heart, Lung and Blood Institute (NHLBI) define metabolic syndrome as the group of risk factors that would increase heart disease and other health problems such as diabetes and stroke. The metabolic risk factors include abdominal obesity, high triglyceride level, low HDL cholesterol level, high blood pressure, and high fasting blood sugar. High sugar diets have been associated with the development of insulin resistance, T2DM, and additional cardiovascular diseases that fall within the realm of metabolic syndrome
[15]. Briefly, these conditions are a result of dietary sugar upregulating hepatic uptake and metabolism of fructose, which leads to liver lipid accumulation, dyslipidemia, decreased insulin sensitivity, and increased uric acid levels
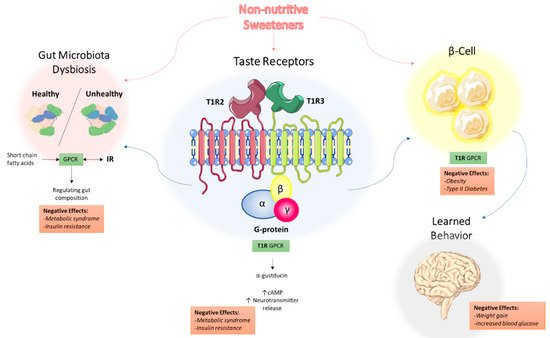
[16]. The role of non-nutritive sweeteners in metabolic syndrome has been discussed in other reviews, with a focus on three potential mechanisms: NNS interacting with sweet taste receptors, NNS interfering with gut microbiota composition, and NNS interfering with learned responses to sweetness
[17]. These three mechanisms are depicted in
Figure 1 and will be the focus of the remainder of this review.
Figure 1. Proposed mechanisms of the underlying effects of non-nutritive sweeteners on the development of metabolic syndrome. NNS interact with the T1R family of sweet-taste receptors through associated G protein α-gustducin, which results in increased intracellular cAMP levels and increased neurotransmitter release. Through the associated GPCR signaling, this may explain how NNS can contribute to metabolic syndrome and insulin resistance. NNS also interfere with gut microbiota composition, with short-chain fatty acids (SCFAs) from dietary intake acting as ligands for GPCRs in the gastrointestinal tract, regulating NNS permeability and gut microbiota composition. Additionally, NNS are associated with insulin and other hormone secretion, which ultimately impact learned behavior and response to sweetness. Abbreviations: NNS, non-nutritive sweeteners; GPCR, G protein-coupled receptor; SCFA, short-chain fatty acid.
5. Non-Nutritive Sweeteners Interact with Sweet-Taste Receptors
5.1. Sweet-Taste Receptors in the Mouth: Perception of Sweetness
The innate universal preference for sweetness once served to support survival as it was associated with food reward and energy (calories) in the form of carbohydrates; however, sweetness is now often delivered via added sugars
[18]. Sweet taste perception first begins at the level of type 2 taste receptor cells (TRCs) clustered in taste buds on the tongue that are G protein-coupled receptors (GPCRs)
[19]. There are two classes of GPCRs that have been identified: the taste 1 receptor (T1R) and taste 2 receptor (T2R) families
[20]. Within the T1R family, the T1R2 and T1R3 subtypes have been found to form heterodimers that act as sweet-taste receptors
[21].
Interestingly, the T1R2/T1R3 receptors recognize all of the chemically diverse compounds that are perceived as sweet by humans, including nutritive and non-nutritive sweeteners
[21]. Given the vast number of compounds that can bind to the sweet-taste receptors, it is not surprising that there are different functional roles of T1R2 and T1R3 with multiple ligand binding sites corresponding to the many possible ligands
[22][23]. Sweet-taste receptor signaling has been extensively studied and reported
[24][25][26]. Since sweet-taste receptors are GPCRs, they can induce the downstream activation of second messenger systems that ultimately result in increased intracellular calcium levels and neurotransmitter release
[23][27]. Briefly, when a sweet-tasting compound binds to the T1R2/T1R3 receptors, α-gustducin is activated. The GPCR Gα-gustducin was previously identified as the first protein molecularly associated with taste cells
[28], but its role in taste signal transduction is still not completely understood. Gustducin has considerable sequence homology to transducin, which is also expressed in taste buds
[29][30]. Both α-gustducin and α-transducin are known to activate a phosphodiesterase (PDE) and decrease intracellular cAMP levels. There is also an increase in phospholipase Cβ2 (PLCβ2) concentration which in turn increases production of inositol 1,4,5-trisphosphate and diacylglycerol. These compounds, in turn, activate the transient receptor potential cation channel subfamily M member 5 (TRPM5), which subsequently increase intracellular calcium and neurotransmitter release
[24][31].
5.2. Sweet-Taste Receptors in the Gut: Effect of Sweeteners on Hormone Secretion
Sweet-taste receptors have also been found throughout the gastrointestinal (GI) tract, the biliary tract, and the respiratory tract, suggesting that non-nutritive sweeteners have additional effects in the body and may not be the inert compounds that they were once thought to be
[23][32][33][34]. Within the GI tract, sweet-taste receptors were primarily found in enteroendocrine L and K cells which secrete specific hormones, as well as in pancreatic β-islet cells
[23][31]. These studies have shown that ligand binding to sweet taste receptors on enteroendocrine cells (EECs) in part affects hormone secretion. In particular, the use of a sweet-taste inhibitor decreased glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) secretion by L cells, without affecting cholecystokinin (CCK) secretion from I cells, which are known to not express sweet-taste receptors
[33][35]. Thus, it appears that this network of sweet taste signaling pathways in the oral cavity and the GI tract mediate the hormonal responses that orchestrate the hunger–satiety cycle
[36].
Enteroendocrine cells comprise 90% of all intestinal epithelial cells and are polarized such that they permit the transport of nutrients from the gut lumen through apical sodium-glucose cotransporter-1 (SGLT-1) and into circulation through glucose transporter-2 (GLUT2)
[37]. The hormones secreted by EECs such as GLP-1, PYY, and CCK can act locally as paracrine factors, neurotransmitters, and neuromodulators, or enter the bloodstream and act as classical hormones at distant sites
[38][39]. It has been established that SGLT-1 based transport is critical for GLP-1 release in humans which enhances glucose-induced insulin secretion from pancreatic β-cells
[37][40]. In animals, several sweet stimuli including NNS have been shown to upregulate SGLT-1 expression and function, suggesting that SGLT-1 activity is modulated by an upstream and broad sweet taste receptor
[41][42]. Thus, it is thought that NNS can potentiate SGLT-1 function and glucose absorption
[43]. NNS including sucralose and Ace-K demonstrate high levels of GLP-1 secretion in in vitro studies, with many inconclusive results in human studies
[44]. Given the collective effects of these hormones, it is likely that they contribute to the pathogenesis of metabolic disorders, including obesity and T2DM
[45][39][46]. Thus, it is possible that NNS can stimulate sweet-taste receptors on intestinal EECs to promote the release of these hormones involved in glucose homeostasis
[18][40].
6. Non-Nutritive Sweeteners Interfere with Gut Microbiota Composition
The gut microbiota consists of millions of bacteria, viruses, and fungi that exist symbiotically within the gut and begins to develop at birth
[47]. The composition and function of the microbiota varies not only amongst individuals, but also changes throughout an individual’s life, affected by external factors such as environmental stressors, antibiotics and diet
[48]. It is thought that diet is responsible for approximately 10% of the influence on intestinal microbiota, a substantial amount when considering the high variability in lifestyle and genetics amongst individuals
[49]. Aberrations in the gut microbiota have been associated with the development of insulin resistance, obesity, and metabolic syndrome; however, the details are still in the process of being understood
[38][50]. In particular, it has been reported that T2DM is associated with alterations in microbiota composition
[51].
In the human gut, the most common phyla are the Gram-positive
Firmicutes and the Gram-negative
Bacteroidetes [52]. Analysis of the gut microbiota in lean and obese individuals has revealed differences in the phyla present. There are several reports on a higher ratio of
Firmicutes to
Bacteroidetes in obese individuals compared to lean individuals, with the proportion of
Bacteroidetes increasing with weight loss
[53][54][55]. As a result, it has been speculated that the differences in the phyla present may be associated with the development of obesity, a component of metabolic syndrome. However, there are conflicting results, and specific roles of phyla have not yet been fully established
[53]. Given the differences in microbiota composition amongst lean and obese individuals and the negligible caloric value of NNS, it is surprising that NNS consumption may induce changes in microbiota composition
[44]. There have been several forms of dysbiosis that have been observed following NNS consumption, mainly an increased ratio of
Firmicutes:
Bacteroidetes and an increase in
Lactobacilli spp., such that the microbiota composition resembles that of obese individuals
[56].
Suez and colleagues first reported the dysbiosis that occurs as a result of NNS consumption in animal studies
[56]. There are several diet-induced animal models of metabolic syndrome, in which the animals are fed a single type or a combination of diets, investigating the whole-body effects of metabolic syndrome such as through hormones, glucose metabolism and lipid metabolism pathways
[57]. Suez et al. reported on the cooperation between microbial species in the gut being linked to enhanced energy harvest that promotes lipogenesis in mice through glycan degradation pathways
[56]. Interestingly, the metagenomes of saccharin-consuming mice were found to be enriched with pathways such as sphingolipid metabolism and lipopolysaccharide biosynthesis, both of which have been associated with T2DM and obesity
[58][59]. Perhaps the most intriguing result of the study was that the
Bacteroidetes to
Firmicutes ratio was positively correlated with reduced glucose tolerance, and the reverse tendency was observed for overweight people, and the deleterious metabolic effects were transferable to germ-free mice
[60]. Thus, it is essential that we consider the gut microbiota composition when developing treatment strategies for T2DM and obesity within the metabolic syndrome platform.
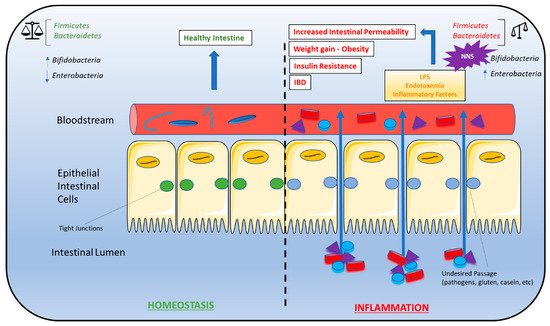
NAS-induced gut microbiota composition changes have been linked to the phenomenon of metabolic endotoxemia, the development of a low-grade inflammatory state by the gut microbiota that ultimately promotes the development of insulin resistance (
Figure 2)
[61]. Briefly, dead bacteria result in the release lipopolysaccharides (LPS) into the gut. LPS is absorbed into circulation where it binds to CD14 proteins (modulators of insulin sensitivity in animals with hyperglycemia, hyperinsulinemia, and weight gain), nucleotide oligomerization domains (NODs), and Toll-like receptors (TLRs) on the surface of the macrophages and dendritic cells. The activation of these innate immune cells initiates several inflammatory processes through the release of inflammatory cytokines
[62]. Overproduction of inflammatory cytokines, in turn, activates additional signaling pathways in metabolic cells that ultimately result in insulin desensitization, altered expression of proteins responsible for glucose transport, increased intestinal permeability, LPS infiltration, oxidative stress, and adipose tissue inflammation
[61]. Metabolic endotoxemia may be a driving force behind NAS-induced obesity and insulin resistance.
Figure 2. Gut microbiota dysbiosis and metabolic syndrome. Dysbiosis of the Firmicutes:Bacteroidetes ratio is associated with several conditions characteristic of metabolic syndrome, including weight gain/obesity, insulin resistance, high-fat diets, gut permeability, and inflammatory bowel disease (IBD). As a result, NNS consumption may contribute to the development of these conditions due to alterations in the Firmicutes:Bacteroidetes ratio. A bifidobacteria decrease combined with an enterobacteria increase leads to endotoxemia that causes a chronic low-grade inflammation associated with some pathological conditions such as insulin resistance and increased gut permeability. A right balance in the microbiota may be considered in gut homeostasis and maintaining the microbiota can be considered prebiotics and restore eubiosis in some pathological conditions. Abbreviations: IBD, inflammatory bowel disease; NNS, non-nutritive sweeteners.
7. Non-Nutritive Sweeteners Interfere with Learned Responses to Sweetness
Sugar and its sweet-tasting nutritive and non-nutritive alternatives have become a staple in the diet. However, sweet-taste has been associated with learned behavior
[63]. As discussed previously, sugar consumption has been associated with an increased GLUT2 and GLUT5 expression, which play a role in CCK expression in the ileum of isocaloric diet-fed rats enriched with fructose or glucose
[64]. The enriched diets provide additional calories, resulting in animals having enhanced total caloric intake
[65]. In contrast to natural sweeteners such as fructose or sucrose, NNS was thought to be excreted after passing through the GI tract unchanged resulting in no energy gain
[66].
Theoretically, the metabolic effects observed with the use of natural sweeteners should be absent with NNS consumption. Paradoxically, NNS consumption has been associated with weight gain. It is hypothesized that the separation of sweetness from calories interferes with physiological responses and the interaction of NNS with sweet-taste receptors in the gut that affect glucose absorptive capacity and homeostasis
[67][68]. Although epidemiological studies have shown an association between artificial sweetener use and weight gain, evidence of a causal relationship is limited; however, recent animal studies provide intriguing information that supports an active metabolic role of artificial sweeteners
[45]. Indeed, the low or zero caloric value of NNS can result in caloric compensation, whereby there is an adjustment for calories consumed at one occasion by reducing caloric intake at subsequent opportunities. Thus, weakened caloric compensation can result in excess energy intake that ultimately leads to increased weight gain
[69].