1000/1000
Hot
Most Recent

Sjögren's syndrome (SS) is a systemic autoimmune disorder affecting approximately 3% of the population in the United States. This disease has a female predilection and affects exocrine glands, including lacrimal and salivary glands. Dry eyes and dry mouths are the most common symptoms due to the loss of salivary and lacrimal gland function. Symptoms become more severe in secondary SS, where SS is present along with other autoimmune diseases like systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis.
Sjogren's syndrome (SS) is an autoimmune disorder of the exocrine glands, including the lacrimal and salivary glands with a strong female predilection; women are affected 10–15 times more than men [1][2]. The disease is present among all age groups but generally starts between ages 40 to 60 years, affecting almost 3% of the population in the United States [3]. The main symptoms of the disease involve a reduction in saliva and tear secretion, leading to dry mouth (xerostomia/stomatitis sicca) and dry eyes (keratoconjunctivitis sicca). The disease can also spread to other organs, leading to various extra-glandular manifestations in the skin, gastrointestinal tracts, pulmonary system, liver, pancreas, kidneys, and nervous systems [4][5]. SS could be primary and secondary. Patients with primary SS show a loss of salivary and lacrimal gland function, while secondary SS develops in patients with other autoimmune diseases [6]. The major cause of illness in SS patients is due to fatigue and joint pain. One characteristic feature of this disease is hypergammaglobulinemia, defined by the presence of tissue-specific autoantibodies, the surge in the levels of immunoglobulins, circulating autoantibodies against ribonuclear proteins (anti-52 and 60-kDa Sjögren's syndrome A; SS-A/Ro, anti-Sjögren's syndrome B; SSB/La), cellular proteins like carbonic anhydrase II, cellular receptors (e.g., β-adrenergic, muscarinic cholinergic), secreted proteins, and detectable Rheumatoid Factor (RF) [7][8]. Production of anti-nuclear autoantibodies (ANAs) and interferon (IFN) are some of the additional features defining SS. Early diagnosis of patients with SS is very challenging and once the diagnosis is confirmed, there are no therapeutic treatments available to treat the disease etiology [9]. It was proposed that aberrant activation of immune cells is responsible for disease progression. However, the detailed mechanism of disease progression in the lacrimal and salivary glands are not determined [10].
Lacrimal gland (LG) is an exocrine tubuloacinar gland that secretes the aqueous layer of the tear film. LG epithelium is composed of three major cell types—ductal, acinar, and myoepithelial cells (MECs). Acinar cells secrete the primary LG fluid, ductal cells modify the electrolyte composition of the primary LG fluid, before it exits the ducts and flows onto the ocular surface and MECs have a contractile function that helps to expel the secreted fluid from acinar cells [11].
The salivary glands are exocrine glands that produce saliva, a mixture of serous and mucous secretions containing water, proteins, glycoproteins, and electrolytes. The salivary glands also produce digestive enzymes that break down different nutrients. Humans have three paired major salivary glands—parotid, submandibular, and sublingual. The parotid glands are the largest salivary glands in humans [12]. Human and rodent parotid glands are composed of pure serous acini, while the human submandibular gland is a mixed gland composed of both serous and mucous acini. In rodents, the submandibular gland is composed of only the serous cells [13]. The acini of human and rodent sublingual glands are composed of mucous and serous cells [14].
Alteration of glandular homeostasis is thought to be an initial event in SS, which happens before the onset of inflammation. Altered homeostasis can also activate the autoimmune response and inflammation. Exocrine dysfunction preceding inflammation was noticed in both mouse models and human patients. Experiments using the NOD mouse model, which is believed to have the same pathogenesis as humans, show an autoimmune phase preceded by non- or pre-immune phases [15][16]. In general, unusual proteolytic activity, high cell death, decrease in expression of the EGF gene, and changes in gene expression levels related to tissue homeostasis are observed before the autoimmune phase [17]. Increased nitric oxide (NO) production was also related to disease pathogenesis in SS patients. NO is generated by nitric oxide synthase (NOS), through the reaction of nitric oxide synthase (NOS) on l-arginine, which produces citrulline and NO [18]. An in vitro study involving mouse and human acinar cells obtained from salivary glands showed that chronic exposure to NO leads to the downregulation of their secretion [19]. Moreover, inducible nitric oxide synthase (iNOS) is a key regulator of the innate immune system [20]. NO is released by vascular endothelial cells and nerves [21] and can induce relaxation of the smooth muscle cells, including pericytes and myoepithelial cells. Decrease in contractile activity of myoepithelial cells leads to salivary and lacrimal gland dysfunction [22][23]. It was reported that in human salivary glands, NOS is localized in ductal epithelial cells [24]. In rat salivary glands, NOS isoforms were found in ductal and myoepithelial cells, while in the lacrimal glands, they localized in ductal and acinar cells. These findings suggest that nitric oxide can directly regulate secretion. In NOD mice, decrease in the salivary gland (submandibular and parotid) function precedes the autoimmune phase and happens in parallel to a decrease in nitric oxide synthase (NOS) activity. This was found prior to proinflammatory cytokine expression or formation of the lymphocytic infiltrations [25].
Further evidence related to the role of non-immune factors in secretory dysfunction was obtained from NOD–SCID mice, where the loss of acinar tissue (mainly due to increased protease activity) happens in the absence of inflammation [26]. It was shown that maintaining acinar cell polarity is crucial for the secretory function of the salivary and lacrimal gland in SS patients [27][28]. Rab3D and Rab8A proteins are required for the exocytosis function of the secretory pathway, and in SS patients it was noted that expression and distribution of the Rab3D protein changed and correlated well with the loss of cell polarity and secretory dysfunction [29]. Another factor that is independent of immune infiltration and linked to SS is high oxidative stress. High oxidative stress leads to overexpression of the reactive oxygen species (ROS) that further causes DNA damage and cell death, leading to a production of anti-DNA autoantibodies. High oxidative stress could lead to SS pathogenesis through ROS production, lipid membrane oxidation, and inflammatory process [30]. High oxidative stress also decreases lacrimal gland secretion by damaging the ocular surface epithelial cells [31], and it is inversely related to the levels of the antioxidant thioredoxin [32][33].
Anomalous activation of the immune pathways leads to disease development in exocrine tissues and systemically to the destruction of epithelial cells (ECs) of the lacrimal and salivary glands. Similar to humans, the lacrimal gland of SS mouse models show periductal and perivascular loci of lymphocytic infiltrates (Figure 1), and loss of acinar and ductal cells, and hence loss of secretory function [34]. More severe destruction of the lacrimal gland was noticed with an increased duration of ocular disease [35]. The most common histological features of the salivary gland of SS patients include loss of tissue structure, acinar atrophy, and hyperplasia of the lining of the intraglandular ducts [36][37]. Several immune cells are implicated in SS progression. We recently reported that in several mouse models of SS, such as MRL/lpr, NOD (NOR/LtJ), and thrombospondin null (TSP1−/−) mice, the majority of cells forming the lymphocytic foci are B cells (Figure 1C,D) [38][39]. Infiltration of the gland involves CD4+ helper T (Th) cells, CD8+ cytotoxic T cells, B cells, plasma cells, macrophages, dendritic cells (DCs), and mast cells [40]. A more detailed analysis of male NOD mice showed the presence of B-cells (52.9%), CD4+ mature T helper cells (14.1%), CD8+ mature cytotoxic T cells (8%), NK cells (8.7%), macrophages (CD11b+ GR1−; 36.5%), and myeloid immunoregulatory cells (4.7%) in the lacrimal gland, indicating a serious inflammatory response [41].
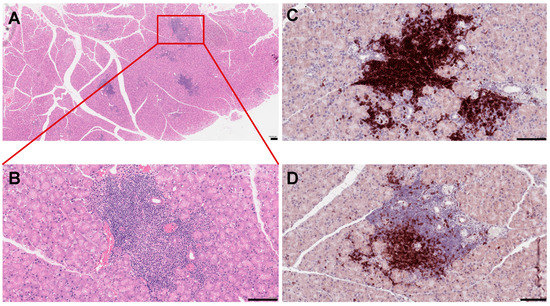
Figure 1. Histopathological features of mouse lacrimal gland at 3 months of age. (A) Histochemical staining of paraffin-embedded mouse lacrimal gland sections with hematoxyllin-eosin (H&E). (B) Higher magnification reveals severe infiltration of immune cells in the lacrimal gland. (C) Immunostaining of the NOD mouse lacrimal gland sections with the B220 antibody (B cell marker) (D) and CD3 antibody (a marker of T cells). Each scale bar is 100 μm.
Symptomatic treatments of SS are the only treatments available thus far, no therapeutic treatment is available to cure the disease [42]. This could be due to the heterogeneity of the disease pathology. Several biological therapies reported in the literature are still in a clinical trial stage [43][44][45][46]. Among all of these therapies, B-cell-targeted therapy showed the most promising results in controlling the SS. Other therapies involving the targeting of T cells and cytokines are still in the early stages of the investigation [47]. Several diagnostic criteria of SS are reported in the literature [48]. The EULAR (see above) promoted a global collaboration to develop an SS disease activity index (ESSDAI) [49]. This activity index measures disease activity in patients with primary SS and is now used as a gold standard in clinical studies [50]. In addition, the EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) [51], provided a questionnaire to the patients that helped to develop ESSDAI, and evaluated systemic complications [50]. Change in ESSDAI is often used as an outcome measure in clinical trials. Ocular dryness can be assessed by Schirmer tests and oral dryness through stimulated or unstimulated salivary flow rate [52]. Here, we discuss various clinical trials that showed encouraging results, and also consider some future targeting therapies for SS-related symptoms.
B cells play a central role in SS disease development and progression due to B cell hyperactivity, GC formation, and the production of SS autoantibodies [53]. Aberrant B cell activation might lead to extra glandular manifestations and changes of other serological characteristics in SS patients, including an increase in levels of free light chains and β2-microglobulin, rheumatoid factor, and hypergammaglobulinemia [7][54]. Ultimately unusual B cell activation might lead to the development of mucosa-associated lymphoid tissue (MALT) lymphoma, in some of the SS patients [55]. Several B cells targeting therapies including the B cell depletion and targeting of BCR signaling (Table 1, Figure 2) are reported up to date.
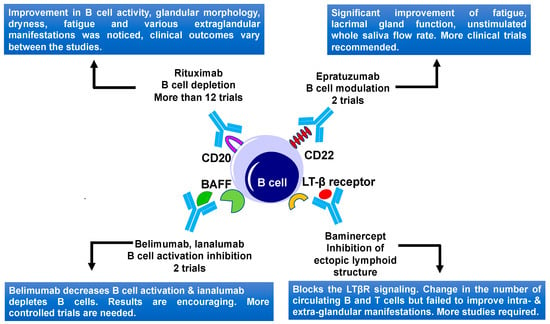
Figure 2. B cell-targeted therapies and their outcomes in primary Sjögren's Syndrome. Current therapies include CD20, CD22, BAFF, and LTβ receptor targeting. BAFF, B-cell activating factor; LTβ, lymphotoxin β; and LTβR, lymphotoxin β receptor.
Table 1. B cell-targeted therapies in SS patients.
|
Drug |
Target |
Dose |
No. of Pats |
Type of Study |
Efficacy |
Side Effects |
Refs |
|||
|
Rituximab |
Chimeric mAb against CD20 |
Twice 1 g on days 1 and 15 |
17 |
Randomized, double-blind, Placebo-controlled pilot study |
Improvement after 6 months, sicca symptoms did not improve |
IRR, SSR |
[56] |
|||
|
|
|
1 g with an interval of 2 weeks or placebo |
30 |
Prospective, single center, randomized, double-blind, placebo-controlled trial |
Stimulated saliva flow rate and lacrimal gland function improvement |
SSR |
[57] |
|||
|
|
|
375 mg/m2/week for 4 weeks or 1 g on days 1 and 15 |
78 |
Prospective study (AIR registry) |
1st cycle efficacy in 47 patients (60 %) After 6 m ESSDAI decrease |
IRR, SSR |
[58] |
|||
|
|
|
1 g with an interval of 15 days. patients received 6 courses of therapy |
41 |
Prospective, multicenter, follow-up study |
ESSDAI decrease. Reduction of infiltrate and GCs after treatment |
No adverse effects |
[59] |
|||
|
|
|
Twice 1 g, 15 days apart |
28 |
Prospective single-center study |
ESSDAI and ESSPRI score improved. |
Not reported |
[60] |
|||
|
|
|
Twice 1 g, two weeks apart |
120 |
Randomized, double-blind, Placebo-controlled, parallel-group trial (TEARS) |
No significant difference |
Few patients had IRR |
[61] |
|||
|
|
|
two doses of rituximab (1 g) or placebo, two weeks apart |
110 |
A randomized double-blind placebo-controlled clinical trial |
No significant difference |
Not reported |
[62] |
|||
|
|
|
Two courses of rituximab (1 g) at weeks 0, 2, 24, and 26 or placebo. |
133 |
A multicenter, randomized, double-blind, placebo-controlled, parallel-group trial |
No significant improvement in any outcome except unstimulated saliva flow |
Few serious adverse events were reported but there were no deaths |
[63] |
|||
|
Epratuzumab |
Humanized anti-CD22 monoclonal antibody
|
4 infusions of 360 mg/m2 biweekly |
16 |
An open-label phase I/II study |
Improvements in fatigue. B-cell reduction, T cells did not change |
Not reported |
[64] |
|||
|
|
|
600 mg every week, or epratuzumab 1200 mg every other week for 4 weeks |
1584 |
Randomized, double-blind, placebo-controlled, multicenter studies |
Disease activity in patients with SLE and associated SS showed improvements |
Adverse events were comparable in the treated and placebo group |
[65] |
|||
|
Belimumab |
Human IgG1ʎ mAb targeting BAFF |
10 mg/kg, monthly dose |
30 |
Phase II open-label |
In 60% of patients improvement in dryness, fatigue, and musculoskeletal pain |
One patient develops pneumococcal meningitis |
[66] |
|||
|
Ianalumab (VAY736) |
a B cell-depleting, BAFF-R blocking, monoclonal antibody |
single infusion at either 3 mg/kg, 10 mg/kg or placebo. |
27 |
Double-blind, placebo-controlled, phase II, single-center study |
Both doses lead to depletion of B cells for a long time |
Moderate infusion related side effects |
[67] |
|||
|
|
BAFF-R |
Monthly s.c. doses (5, 50, 300 mg) or placebo. |
190 |
Phase 2b Study |
Primary endpoint achieved, improvement for 300 mg dose |
Safety profile looked good |
[68] |
|||
|
Baminercept |
Lymphotoxin-β receptor Fusion protein, reduces B cell infiltration |
s.c. injections of 100 mg of baminercept every week for 24 weeks or placebo |
52 |
Phase II multicenter, randomized, double-blind, placebo-controlled trial |
No significant difference in ESSDAI, no difference in salivary gland secretion and ocular dryness |
Higher incidence of liver toxicity |
[69] |
|||
This table displays B cell targeted therapies for SS. The table displays drugs and drug's dose, targets number of patients (Pats), study type, efficacy, and side effects. Abbreviations: mAb—monoclonal antibody, CD20—cluster of differentiation 20, IRR—infusion-related reaction, SSR—serum sickness-related, AIR airway intervention registry, ESSDAI—the EULAR Sjögren's syndrome disease activity index, ESSPRI the EULAR SS patient reported index, MSG—minor salivary gland, TEARS -tolerance and efficacy of Rituximab in primary SS, SLE—Systemic lupus erythematosus, BAFF—B-cell activating factor.
It was proposed that T cells form a major part of the lymphocytic infiltrates in salivary and lacrimal glands, which mainly consists of CD4+ T cells at the early stage of the disease [70]. Interaction between activated CD4+ T cells and B cells, play an important role in B cell hyperactivity in SS patients. Therefore, targeting T and B cell interaction could be a powerful approach to treat the SS [71]. Abatacept is a human fusion molecule where the Fc region of human IgG1 is attached to human cytotoxic T lymphocyte antigen 4 (CTLA-4) protein (Figure 3). It prevents CD28-mediated T cell co-stimulatory signal, by blocking the crosstalk between the antigen-presenting cells and the T lymphocytes. Abatacept blocks the interaction of CD80/86 present on APC with the CD28 ligand, which is located on the surface of T cells, which is important for T cell proliferation and cytokine production [72]. In the open-label study, Alder and coauthors [73] demonstrated the effectiveness and safety of abatacept in patients with early stages of SS. Treatment of primary SS patients with abatacept led to a reduction of inflammation of the salivary gland and an increase in saliva production. Moreover, this treatment led to a significant increase in circulating B cells in the blood and a reduction of Treg frequency in the salivary glands. An increase in saliva production was comparable to rituximab therapy [73]. In another abatacept treatment study, 15 SS patients were treated with 8 doses of drugs. During the abatacept treatment, a significant reduction in ESSDAI, ESSPRI, rheumatoid factor, and IgG levels were noticed, but these factors increased again when the treatment stopped. However, the function of salivary and lacrimal glands did not change significantly during the treatment, whereas fatigue and quality of life improved significantly [74]. Abatacept was also found to be effective in a study involving 36 patients with SS associated with rheumatoid arthritis. Results showed an improvement in salivary and lacrimal gland secretory function, as well as the sicca symptoms [75]. Verstappen and coauthors [76] studied the effect of abatacept on the homeostasis of CD4+ T cell and B cell subsets, as well as on T-cell-dependent B cell hyperactivity in SS patients. They noted that abatacept reduces the number of circulating follicular helper T (Tfh) cells and Treg cells, whereas it had no effect on other CD4+ effector T cell subsets. Circulating CD4+ T cells decreased the expression of the activation marker ICOS, after treatment with abatacept. Lower ESSDAI scores of SS patients during treatment correlated well with reduced ICOS expression in the Tfh cells and decreased Tfh cell number [76]. To understand the effect of abatacept treatment on the histopathological changes of the parotid gland of SS patients, a pilot study involving 15 SS patients was performed [77]. No decrease in lymphocytic infiltrations, focus score, and number of CD20+ B cells were observed. However, a reduction of GCs was noticed with abatacept treatment, as the formation of GCs rely on co-stimulation of Tfh cells [77]. These studies revealed the importance of the early stages of SS treatment and hence the importance of early diagnostics. These studies also implied that early diagnosis can play a decisive role in choosing treatment strategies and improving the quality of life of SS patients.
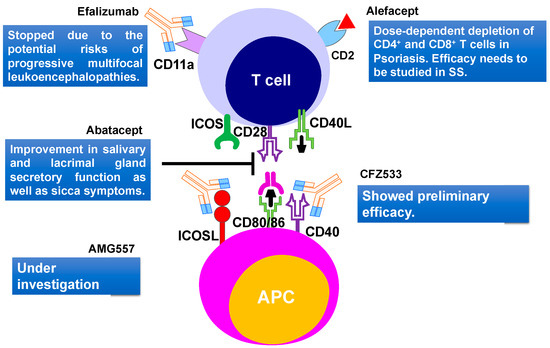
Figure 3. T cell-targeted therapies and their outcomes in primary Sjögren's Syndrome. Current therapies include CD40, CD80/86, ICOSL, CD11a targeting. CD2, cluster of differentiation 2; CD40, Cluster of differentiation 40; CD28, Cluster of Differentiation 28; CD80, Cluster of differentiation 80; ICOS, inducible costimulatory; ICOSL, ICOS ligand.
A phase III clinical trial involving 80 SS patients with early and active stages of the disease evaluated the efficacy and safety of abatacept in primary SS patients. The study revealed no significant difference between the treated and the non-treated groups, based on the primary outcome ESSDAI score. Further studies need to be performed to conclude whether the abatacept treatment is beneficial for a specific group of SS patients [77].
Another humanized monoclonal antibody efalizumab, that targets the adhesion molecule CD11a subunit of leucocyte function-associated antigen-1 (LFA-1) was recently developed (Figure 3). The LFA-1 is thought to be involved in indirect interaction with T cell activation and reactivation [78]. This drug was initially approved for psoriasis and showed positive effects in TNF-α refractory patients [79]. The use of efalizumab trial in SS patients started in 2006 but stopped due to the potential risks of progressive multifocal leukoencephalopathies [80]. Efalizumab was withdrawn from the market in 2009.
Cell surface glycoprotein, CD2 is present on T cells and is important in T cell adhesion and activation. A dimeric fusion protein Alefacept binds to the lymphocyte antigen CD2, inhibiting the leukocyte function-associated antigen-3 (LFA-3) and CD2 interaction interfering with the T lymphocyte activation. This protein was also used in psoriasis treatments [81]. A major concern for therapy was the dose-dependent depletion of CD4+ and CD8+ T cells [82]. A recent trial of alefacept involving 15 mg i.m. per week in type 1 diabetes showed encouraging results without major adverse effects [83]. Due to the similarity with the SS pathogenic mechanism involving memory T cells, this drug should also be studied further for the treatment of SS (Figure 3). Few other biological therapies like anti-CD40 treatment with CFZ533 (NCT02291029) [83] and anti-ICOSL treatment with AMG557 (NCT02334306) [83] are still under investigation (Figure 3).
The M3 muscarinic acetylcholine receptor (M3R) is a G protein-coupled receptor that is expressed in salivary and lacrimal glands and plays a central role in exocrine gland secretion [84][85]. Autoantibodies against lacrimal gland M3R, resulted in a primary, organ-specific dysfunction [86]. Thus, M3R could be a potential target for treating SS. M3R reactive T cells were found in the peripheral blood of SS patients. A study on Experimental Sialadenitis-like SS mice was performed using T cell epitopes of the M3R. In this study, cytokine production by M3R-reactive CD4+ T cells in response to M3R peptide was analyzed. Results showed the production of mainly interleukin-17 (IL-17) and interferon-γ (IFN-γ), in response to the M3R peptide. Altered peptide ligands for T cell epitopes designed for this study suppressed sialadenitis in vivo, through the induction of anergy and significantly suppressed IFN-γ production in vitro [87]. This indicates the potential of this strategy in controlling pathogenic T cell infiltrations in the glandular tissue of SS patients.
As T cell-dependent B cell hyperactivity plays a crucial role in SS pathogenesis, targeting crosstalk between T and B cells could be a successful approach to treat SS. Therefore, treatments of the early stages of SS could be more beneficial than treatments of late stages.
Mesenchymal stem cells (MSCs) are multipotent stromal cells with the ability of self-renewal and differentiation [88]. They are derived either from bone marrow (BMMSCs), umbilical cords (UCMSCs), gingiva MSCs (GMSCs), or adipose tissues (ADMSCs). MSCs are able to modulate the immune response of various immune cells, including dendritic cells (DC), macrophages, natural killer T cells (NKT), and mast cells (MC), which might also affect the pathogenesis of SS [89]. It was shown that the treatment of SS patients with MSCs improves sialadenitis, mainly by causing a decrease in Th1, Th17, and Tfh cells, and an increase in the number of Tregs [90][91].
The Tfh cells play an important role in B cell maturation and differentiation. Umbilical MSCs could be used as a novel therapeutic approach (Table 2) in SS patients, due to the production of indoleamine 2,3-dioxygenase (IDO) [90]. IDO converts tryptophan into kynurenine, thereby suppressing the T cell proliferation, while inducing differentiation of naïve T cells to FoxP3+ T regs [90][92]. At the same time, Alunno and coauthors found that human UMSCs had no effect on T cells implicated in SS, but just prevent the proliferation of healthy T cells [93]. Though UMSCs have a great therapeutic potential, it is difficult to use them in the transplantation approach, due to the systemic immune response in the host. In this context, a microencapsulation technique that separates UMSCs and T cells was developed [93]. These microencapsulated UMSCs restore the Tregs/Th17 ratio and suppress SS T cell proliferation [93], indicating the importance of a drug delivery platform in modulating the immunomodulatory effects of UMSCs in SS. In order to understand the mechanism of human UMSCs and their effect on SS pathogenesis, UMSCs were administered to NOD mice, prophylactically and therapeutically [94]. Tregs upregulation was noted showing the potential of UMSCs in treating SS [94].
Table 2. Stem cells targeted therapies in pSS patients.
|
MSCs |
Cell Number, Origin |
Administration |
Effect |
Refs |
|
UMSCs |
1 × 106 /Kg one dose |
iv |
Increase saliva flow, reduction in anti-SSA/Ro and anti-SSB/La antibodies |
[91] |
|
UMSCs |
Human N/A |
Coculture |
Differentiation and proliferation of Tfh cells decreased |
[90] |
|
UMSCs microencapsulated |
Human N/A |
Coculture |
Decrease in proliferation of T cells, and numbers of Th1, Th17; Treg increased |
[93] |
|
UMSCs |
Human 1 × 106 /Kg |
iv |
Reduced IL-12, decrease in Th17 and Tfh cells; Treg increased |
[95] |
This table displays SS therapies targeting mesenchymal stem cell (MSC). The table shows the origin of the MSCs, injected cell number, route of administration, effect of treatment, and references. Abbreviations: UMSCs—Umbilical cord-derived mesenchymal stem cell, iv—intravenous, SSA—Sjögren's syndrome A antibodies, SSB—Sjögren's syndrome B antibodies, Tfh—T follicular helper, Th1—T helper type 1, Th17—T helper 17, Treg—T regulatory cells, and IL-12—Interleukin-12.
Moreover, it was shown that cultured human UMSCs have the potential to differentiate into salivary gland epithelial cells. Thus, BMSCs co-cultured with the salivary gland epithelial cells develop comparable cellular structures like tight junctions and secretory granules, and also showed an increase in various salivary gland genes such as aquaporin 5, E-cadherin, and α-amylase [96][97]. This suggests that BMSCs transplantation is a promising therapy to treat SS by replacing damaged salivary gland acinar cells. However, a relatively recent study in mice in which BMSCs were delivered systemically through an i.p. injection, showed that, despite a positive temporary effect of this treatment on the lacrimal gland function, BMSC did not engraft into the epithelial component of the gland [98]. Several other studies also reported the therapeutic benefits of MSCs, even though engraftment of these cells into the epithelial compartment of the lacrimal gland was not detected [99][100].
The IL-12 level is known to increase in many autoimmune diseases and thus could be a potential target for SS treatment. Bingyu and coauthors analyzed the effect of MSC transplantation on IL-12 production, through dendritic cells in 29 SS patients. They found that DCs from SS patients produced more IL-12 compared to the control patients. Upon MSC transplantation, a reduction in IL-12 and an increase in saliva flow was noticed. MSCs also increased the number of Tregs and downregulated Th17 and Tfh cells [95].
Another study involving 404 patients with different autoimmune diseases, like SLE, SS, and rheumatoid arthritis was performed. Patients received allogeneic mesenchyme stem cell infusions and were evaluated for adverse events, to make sure that MSCs are safe to use as a treatment of autoimmune disease [101]. This study suggests that MSC infusion is a safe therapy for patients with autoimmune diseases.
In summary, MSCs have a potent immune-modulatory function because they suppress Th1/Th17/Tfh cell responses and upregulate Tregs. They can also modulate the function of DC, macrophages, mast cells, and NK cells. Due to their effect on the adaptive and innate immune system, MSCs could be used as a potential therapeutic treatment option for some SS patients. However, additional clinical trials are necessary to further understand MSC's therapeutic potential for SS patients.
Cytokines constitute a complex signaling network and their dysregulation might lead to systemic or exocrine gland disorders. There are 38 interleukins (ILs) reported so far that could be used as potential therapeutic targets to treat several autoimmune diseases [102]. A list of cytokines that were used as a therapeutic target in SS is summarized in Table 3 and Figure 4. The majority of cytokine-targeted therapies in SS include the TNF family, IFN family, IL-1, IL-2, IL-6/12, IL-10, and IL-17 [103].
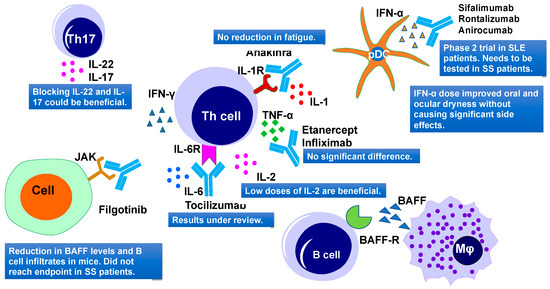
Figure 4. Cytokine-targeted therapies and their outcomes in primary Sjögren's Syndrome. Current therapies include IL-1R, TNF-α, IL-6R, IL-2, JAK, IFN-α targeting. IL, interleukins; TNF-α, tumor necrosis factor–α; IFN-α, interferon α; and JAK, Janus kinase.
Table 3. Cytokine-targeted therapies in pSS patients.
|
Drug |
Cytokines |
Target |
Dose |
No of Pats |
Phase of Study |
Efficacy |
Side Effects |
Refs |
|
Infliximab |
TNF family |
TNF-α |
3 mg/kg two weeks apart, three infusions |
16 |
Phase II |
Improvement in the visual analog score, fatigue, and dryness |
No significant adverse events were seen |
[104] |
|
infliximab |
TNF family |
TNF-α |
3 infusions of 5 mg/kg drug or placebo two weeks apart |
103 |
Randomized, double-blind, placebo-controlled study |
No significant differences |
Severe adverse events reported in the infliximab group |
[105] |
|
Etanercept |
TNF family |
TNF-α |
25 mg s.c. twice per week for 12 weeks |
15 |
Pilot study |
No increase in salivary or lacrimal gland function |
Injection-site reactions occurring in about one-third of patients |
|
|
IFN-α |
IFN-α |
|
150 IU of interferon-α 3 times a day for 24 weeks |
12 |
Double-blind placebo-controlled |
Improvement in symptoms of xerostomia and xerophthalmia |
Well tolerated |
[108] |
|
IFN-α |
|
|
150 IU of interferon-α 3 times a day for 24 weeks |
497 |
2 Phase III clinical trials |
Majority of symptoms improved |
No significant adverse effect noted |
[109] |
|
Tofacitinib |
IFN |
|
0.0003–0.005% daily |
327 |
Phase 1/2 prospective, randomized |
Better patient-reported ocular tolerability |
Well tolerated |
[110] |
|
Anakinra, a non-glycosylated recombinant version of the human IL-1 receptor antagonist, IL-lRa |
IL-1 |
IL-1R blockade |
100 mg/day or a placebo for 4 weeks |
26 |
A double-blind, placebo-controlled parallel-group study |
No significant changes |
Two serious adverse events (SAE) were observed |
[111] |
|
Tocilizumab |
IL-6 |
anti-IL-6 mAb |
8 mg/kg |
1 |
Case study |
EULAR SS activity Index was stabilized at 4, CT scan and pulmonary function normalized |
Treatment was well tolerated |
[112] |
This table displays the cytokine-targeted therapies for Sjögren's syndrome reviewed in this article. The table displays cytokines and their target including drugs and their doses, number of patients, phase of study, efficacy, and side effects. Abbreviations: TNF Tumor necrosis factor, s.c. subcutaneous, IU International unit, IFN Interferon, IL-1 Interleukin-1, IL-1R Interleukin-1 receptor, and CT scan Computed tomography scan.