1000/1000
Hot
Most Recent

Three-dimensional (3D) printing offers significant potential as an efficient fabrication technique on personalized organs as it is capable of biomimicking the intricate designs found in nature. In this review, the determining factors for hip replacement and the different fabrication techniques such as direct 3D printing, Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS) and stereolithography (SLA) for hip replacement. The study also covers surface modifications of 3D printed implants and provides an overview on 3D tissue regeneration. To appreciate the current conventional hip replacement practices, the conventional metallic and ceramic materials are covered, highlighting their rationale as the material of choice. Next, the challenges, ethics and trends in the implants’ 3D printing, outlook and challenges are also presented.
Annually, the number of people globally experiencing pains from organ failure or dysfunction from a devastating tissue is on the rise, and this usually affects children and the ageing population. Traumas or illnesses, which include strokes, joint degeneration and heart attacks, can adversely reduce the life quality of the victims and lead to the significant damage of the tissues where new medications are incapable of efficient healing [1].
The hip joint comprises two bones which are the femur (thigh bone) and pelvis as illustrated in Figure 1. This joint is the biggest ball and socket synovial joint in the body. This ball is the femur head, which is the rounded edge of the femur while the socket is a curved dip around the lower region of the pelvis (acetabulum). The femur headsets in the pelvis give shape to the hip joint.
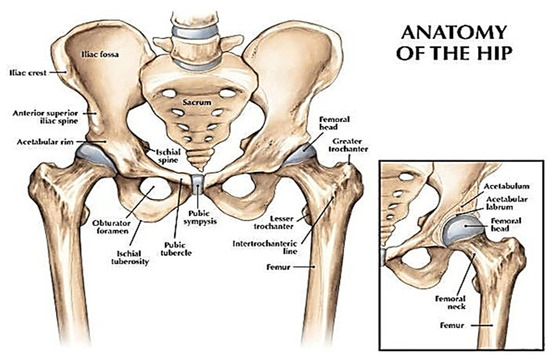
Figure 1. A view of the hip joint [2]. Reprinted with permission from Ref. [2]. Copyright 2007, Lippincott Williams & Wilkins, Inc.
Hip pain is common, and it can affect anyone at any age; however, it is most common with the elderly [3]. For instance, in the United States, 76 million people are experiencing some type of pain, and this is a sizeable chunk of the population [1]. It is difficult to pinpoint the specific causes of these pains, but the clues lie in the type of pain and the location of the pain. For most cases of the hip, the pains relate to any problem emanating from the lower back or buttocks region. Meanwhile, the hip problems are relative to the source of the pain in the groin, thigh region and in some cases, the knee because of the hip innervation when the nerve is stimulated [3]. In addition, in the United Kingdom, the National Health Services (NHS) has been supervising the National joint registry for England, Wales, Northern Ireland and Isle of Man (NJR) for all forms of hip surgeries. From the NJR review of 2016, about 101,651 replacements were done that single year which was roughly a 3.5% increase from the previous year [4].
The several conditions which lead to hip pains can be a result of the following: arthritis, injuries, pinched wounds, cancer and other problems such as lifestyle [5]. Arthritis, in particular, is a broadly known condition which refers to pain and inflammation in a joint according to NHS UK and this is mainly due to more than 10 million people currently have this condition which affects people of all ages not excluding children. The cartilage, although flexible, is a firm connective tissue in the joint. The joints are protected when the cartilage absorbs the shock and pressure created when there is movement and stress is applied to them. Cartilage tissue reduction in standard amounts can lead to various forms of arthritis. Osteoarthritis (OA), which is the known form of arthritis commonly caused by normal wear and tear and an injury/infection, will most times aggravate the breakdown of the cartilage tissue.
Non-surgical treatments are considered the first line of action during the treatment of hip pains. Most of the hip replacement implants (90–95%) are durable for at least 10 years; there is a rising public demand for increasing the life span of these implants based on increasing life expectancy amongst the older population with joint degeneration illness [6][7]. As a result, revision hip arthroplasty could be considered when the implant fails due to several reasons such as the aseptic loosening (51.9%), instability (16.9%), infection (5.5%), debilitating pain, periprosthetic fractures or component failure [8]. In addition, there is a limitation on the conventional 2D scans currently, which frequently does not provide well-detailed imagery during implantation. However, this review focuses on the current state of 3D printing technology and the required materials needed to support cell adhesion and growth. Therefore, at this point, a brief review of real-life application of 3D printing needs to be provided.
Hip surgery involves hip replacement and an alternative surgical option for hip arthritis. When the treatments and medications applied do not nip the hip pains to a suitable level, this surgery for reposting or replacement of the hip joint may be the next solution.
In comparison with the conventional technologies which are solely CT and MRI imaging, a 3D model provides more details for the team and can be also be used by the MDT to simulate the operation. Generally, two main ways 3D printers can be used in hip surgery are either as implants or models. 3D printed implants can be made from suitable materials which are ideal for the reconstruction of huge and unclassifiable acetabular defects. 3D printed models of patients can assist in planning surgery, delivering surgery and teaching surgery [9].
There has been a significant improvement in bioimplants in the current decade, where a wide variety of fabrication methods are being applied. In 2018, the global orthopaedic implant is estimated to be at USD 4.747 billion and a compounded annual growth rate (CAGR) of 5.1% is expected from 2019 to 2026 as depicted in Figure 2 [10].
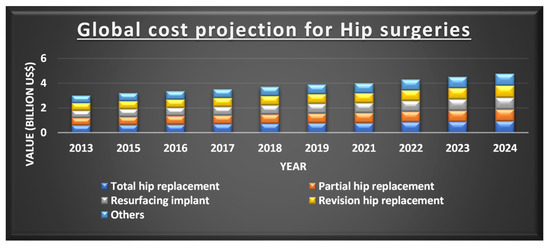
Figure 2. Projection of the cost for different types of hip implants. Reprinted from Ref [10]. Copyright 2019, Polaris Market Research.
In the human body, the biological functioning is complicated, with the huge differences in biomechanical properties from bone to bone. Such an instance is the elastic modulus of the critical section of denser bones varying from 16–20 GPa; this is a magnitude greater than the trabecular bone. Therefore, it can be understood that certain biomechanical errors are bound to happen between the recently implanted parts and closer bones with similar properties. Furthermore, from a medical perspective, these biomechanical properties may differ greatly from the body to the body. Hence, a need for fabrication techniques that can meet specific geometry for a precise injury/defect is justifiable. Additive manufacturing (AM), also termed rapid prototyping (RP) technology, is a common name for the fabrication technique depending on the idea of surface development. From its emergence in the 1980s, this technique has been garnering research interest in the sector of manufacturing [11][12][13][14]. In contrast to conventional implants, 3D printed implants can be tailored to several forms of diseases [15][16][17]. With the possession of excellent design ability, 3D printed implants can solve certain challenges where it is complicated to insert and repair the different conventional implants together [15][16][17].
The pros of 3D printing include the capability of biomimicking extracellular matrix (ECM) and the ability to fabricate adaptable scaffolds regardless of the shape complexities for the cell distribution to done homogenously. However, the major limitation is the accessibility of suitable biomaterials that possess the stability and intrinsic properties for 3D printing of scaffold. An additional limitation is a time required for scaffold fabrication, and that time increases when the design becomes more complex and accurate [18]. It is worth noting that 3D printers utilize varying powdered mixtures and materials; the size of the structures can easily affect the printability of the scaffold for most materials in 3D printing. For a material to be a viable choice for tissue regeneration, it should be printable with a great degree of reproducibility from 3D printing. These materials should be affordable, effective and malleable to create the morphology required for the designed scaffold. Within the last four decades, various 3D Printing (3DP) techniques have been suggested due to the processing approach. Of importance is the ASTM/ISO 52900:2015 standard [19][20] designated over 50 different 3D techniques which can be grouped as (i) binder jetting, (ii) direct deposition, (iii) material extrusion (FDM), (iv) material jetting/inkjet, (v) powder bed fusion (SLS), (vi) sheet lamination, (vii) stereolithography (SLA, DLP). For this review, the emphasis is on direct 3D techniques which usually utilize several forms in atmospheric conditions such as fluids capable of solidifying, nano fine powdered particles, layered sheets and flexible filaments.
Currently, 3D printing products do not have a formal legal standing that clarifies them both for implantable and non-implantable devices. Using Europe as the base reference, whole 3D-printed products can be classified as customized tools under the regulation (EU) 2017/745 of the European Parliament and of the council of 5 April 2017 [21][22]. It was stated that “any device specifically made in accordance with a written prescription of any person authorized by national law by virtue of that person’s professional qualifications which gives, under that person’s responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs”. Varying from mass-produced devices “which need to be adapted to meet the specific requirements of any professional user and devices which are mass-produced by means of industrial manufacturing processes in accordance with the written prescriptions of any authorized person shall not be considered to be custom-made devices” [22]. In fact, manufacturers of customized tools are only assured through a commitment of conformity assessment methods whereby the tools must comply with the performance and safety requirements [23].
The most regularly used 3D printing techniques include direct inkjet printing, bioprinting, powder deposition printing (FDM), laser-assisted printed (SLS) and stereolithography (SLA). Therefore, the study aims to discover methods for novel biomaterial fabrication through 3D printing that can be used in hip implant design, applications and is a biocompatible tissue scaffold. Before the AM technique, 2D slice data are obtained from the designed surface of 3D structures. Required materials are fabricated through the combination of material layers [24]. As opposed to conventional fabrication techniques that take out materials from a whole, AM technique creates 3D materials by continuously adding layers instead.
The bone is the second most-transplanted tissue in the world, with over four million operations using bone grafts or bone replacement materials and these includes hip replacements. The demand for this type of operations is constantly growing. Therefore, the development of bioactive three-dimensional scaffolds (3D) supporting bone regeneration has become an important area of interest in bone tissue engineering (BTE), including the 3D printing method of increasing importance. It should be noted that individual groups of materials, including polymers, ceramics and hydrogels, are not able to reproduce bone properties when used alone fully. However, when groups of materials are used together in 3D composite scaffolds, research shows that you can get beneficial properties and improve bioactivity. Bone is a heterogeneous composite material consisting of hydroxyapatite, type I collagen, lipids, non-collagen protein and water. Therefore, during the production of scaffolds, it is advisable to use a composition of materials to obtain a composite scaffold, and thus potentially enabling greater scaffold bioactivity and structural biomimicry. The bioactivity of the scaffold is also increased by the inclusion of materials that can interact with or bind to living tissues.
On the other hand, increased scaffold bioactivity can lead to better bone cell ingrowth (osteoconduction process), stable anchoring of scaffolds in bone tissue (osseointegration process), stimulation of immature host cells to transform into osteogenic cells (osteoinduction process) and increased vascularization. A perfect 3D scaffold should consist of a biocompatible, biodegradable material with similar mechanical properties to the tissue in which it is to be implanted. The scaffolds are not intended for permanent implants and ideally facilitate host cell deposition of the extracellular matrix (ECM) and replace the scaffold structure over time. Therefore, the 3D architecture of the scaffolding should be very porous with the connected structure to facilitate cell attachment, proliferation and differentiation [25].
Tissue engineering, as it is known currently, is a multidisciplinary field that applies the concept of life sciences and engineering towards the continuous development of natural alternatives. This has rapidly evolved from the area of biomaterial development and entails the process of combining cells, scaffolds and bioactive molecules into functional tissues. The objective of tissue engineering is to gather a functional structure that can repair, preserve and enhance tissue functionality or the whole organ [26]. On the other hand, regenerative medicine/tissue regeneration is a broad field which includes tissue engineering. In addition, it integrates advancement in self-healing, which is a situation where the body uses its system, most times with the aid of foreign biomaterials to reproduce cells and restore tissues and organs.
The goal of tissue regeneration through surgery is to replace damaged/diseased tissues with healthy and performing tissues, tissue regeneration tends to focus on the cure rather than treating complex, often incurable diseases. This has been made possible through tissue engineering, which requires extensive knowledge of the biological process necessary for differentiation and proliferation at the cellular level. This tissue engineering process often starts with a scaffold which is a 3D structure support material required for the suitable differentiation and proliferation of the cells immersed in the scaffold.
The area of tissue engineering and regeneration seeks to address these vital statistics for an improved implant application. This originally involved the transplanting of tissue from one area to another within the same body (Autograft) or from one body to a different body (an allograft) has been effectively used in replacing organs with consistent results [27].
However, there still exist multiple problems with both procedures (autograft and allograft). The autograft technique is expensive and might cause an increased risk of infections, additional injury and is limited due to the unsuitable anatomical replacements from a different body region. While allografts are often not fully accepted by the immune system (immunosuppressant therapies) which is necessary and pose a threat from infection risks and may lead to the possible transfer of illnesses or diseases between the bodies. Most recently, there has been a spike in the study of tissue replacement designs that utilize physical, biological or/and mechanical components to restore functionality [28][29][30][31][32]. Specifically, tissue engineering originally involved the concept of cell isolation from a body, proliferating in vitro and growing them into a biomaterial that is subsequently implanted into the spot of the injury via in vivo. As such, the goal is to fabricate artificial tissues and organs to seek redress for the reduction of risks from the grafting methods (allograft an autograft). Several developed studies supply the needed information regarding how the cells interact with the extracellular matrix hip (ECM) to determine cell behaviour and function [33][34][35][36][37]. ECM in a 3-dimensional structure provides the mechanical support for cells around it. The potential for synthetic biomimicry mechanism development like ECM is an advantage of tissue engineering. A benefit of using 3D printers in replacement is that in certain biomaterials such as thermoplastics, cells can be inserted at the right temperature and precise location to produce a 3D implant that is based on the obtained clinical imaging. Through this process, a strong hip implant is produced that is surgically inserted to heal the bone/tissue deformities, and this implant would biologically degrade with time to leave behind solely natural bone/tissues.
The biomaterials to be used for tissue engineering should have the following features [37]:
(i) Should be porous (to ensure nutrient movement, removal of waste and cell growth), biocompatibility, reproducibility, cell/tissue compatibility, easy preparation and biodegradable.
(ii) Lead to the reduced inflammatory reaction, therefore, decreases the possibility of immune system rejection.
(iii) Advantageous if the biomaterial tissue scaffolds can act as substrates that support cellular fastening, growth and differentiation.
(iv) The cells grow and differentiate, and this scaffold must have the ability to resist the forces put in by the cells else the scaffold disintegrates and causes dismal diffusion of nutrients, waste and oxygen.
(v) The scaffold structure should be mechanically stable to be capable of maintaining load-bearing and varying body movements in daily activity on the joint.
Hip replacement has witnessed a rapid advancement over the past decades, and the specific techniques for surgery have evolved. With the continuous advancement of hip replacement, the biological knowledge of orthopaedic tissues continues to advance. Likewise, the demand for biological solutions for pre and early defective hip remains a challenge for surgical hip treatment [38]. Furthermore, within the hip, there is a rich presence of vascular tissues, and this results in complexities during hip replacement surgery as adjacent vessels could be damaged during operation, an efficient preoperative planning procedure would significantly prevent this [39]. Other vital orthopaedic tissues include the articular cartilage, labral fibrocartilage and Ligamentum Teres [38].
Tissue preservation or minimal invasive total hip replacement (THR) is currently becoming a priority with the focus being to reduce hospital stay, improve rehabilitation and faster patient recovery [40]. The regenerative process replaces and renews the stem cells to facilitate the preservation, restoration and re-establishment of optimum functionality for tissues and organs. At the early stages of some hip defects, simple injections of stem cells to the hip can position it to regenerate and heal the damaged tissue and bone cell lines [41]. The progress noticed in drug testing and regenerative therapy can significantly benefit from bioengineered human tissues developed via several cell types with precise 3D structure. However, there is a limitation with the production of human tissues which are greater than the millimetre size, and this is due to a lack of techniques for fabricating tissues with embedded life-supporting vascular networks [42].
In brief, the important role in hard tissue engineering and regeneration is played by intelligent biomaterials and structures that exert an instructive/inductive or triggering/stimulating effect on cells and tissues by engineering the material’s sensitivity to internal or external stimuli (e.g., pH, temperature, ion strength and magnetism, favouring the repair and regeneration of damaged tissue). The second group is biomaterials that exhibit intelligently tailored individual properties and controlled functions to actively participate in tissue regeneration in a valuable way.
Although there is an improvement of 3D printing that has led to the scaffold fabrication in nanoscales which has varying tissue applications, this process of adopting 3D printing for clinical applications is lagging. Recent obstacles to the development are in the aspect of biological, cost, engineering and administration/safety [32]. For bioactivity, it is required that the necessary activities of the cells, which include cell migration, oxygen diffusion and vascularization grades, should be considered due to the below-par performance. For engineering, the reproducibility and producibility of the scaffolds must be required to establish a homogenous and consistent utilizations.
As 3D printing is increasingly utilized in several orthopaedic applications, this technology must be reliable and consistent in delivering high-quality implants which are needed. Therefore, the quality assurance of these implants is crucial. There are still various challenges facing the implementation of quality control measures and they are discussed in this section. To ensure 3D printed implants meet the required standard, the quality of the material used is an important factor. However, most manufacturers are experiencing difficulties with material qualifications. A major factor that is making it complex is maintaining purity of powdered material used in additive manufacturing. It is very easy for powders to get contaminated. Another challenge is when leftover powders during printing are reused. Although reusing can assist in waste reduction, it is worth noting that repeated reuse of the powders like this can alter the particle composition because there is a likely absorption of moisture, nitrogen and oxygen, there is also a possibility of oxidation. Therefore, testing procedures for 3D printing is necessary for ensuring that there is no contamination. CT scans have been suggested as a precise method of detecting contaminations in powders as it provides a useful and robust imaging processing methods which generate detailed reports on material porosity, pore and particle morphology and also shape and size distribution of particles. This is useful in validating raw materials.
Another challenge is the broad range of variables from the 3D printing technique that can influence the structure and form of the implant. These variables cover the total 3D printing workflow, which spans from design to the fabricating and post-processing. Some of these variables are the design of the support structure and the number of times for powder reuse. Currently, manufacturers use a trial and error approach to handle the various variables to obtain a technique that can reproduce implants. However, these may lead to the manufacturing of the end product multiple times and requires extensive testing of the implant. A closed-loop quality control system has been suggested as the solution for this anomaly, for this the integration of three elements is required for a more reliable, faster and sustainable quality assurance for a 3D printed implant and the elements are planning the structure through simulation, in-process monitoring of the build process and feedback control that spots deviations of parameters during printing and automatically repairs the structure to balance them. Finally, human error is another challenge which poses the biggest risk in ensuring 3D printed implant meets the necessary standard required. This can be attributed to 3D printing still needing more human intervention than is expected, and this comprises the design, support removal to post-processing and the manual inspection requirements to converge at each stage. With the level of human interventions, it unavoidably increases the risk of compromising the end product. Therefore, quality management is essential in this area [39][40].
3D printing is still a relatively emerging process, and a lot of certifications and standards are still being developed. For the future, the industry needs to have to develop protocols, reference data and testing methods to reduce the time and cost for the qualification of 3D printed implants and processes. As of now, certain standards have been developed for classifying and qualifying the machines and processing techniques of 3D printing such as ASTM/ISO 52900:2015 and ASTM F3303, but they do not cover bioimplant applications. The next phase of this development is quality assurance, support of the implant qualification and post-processing of the printed implants. In addition, the National Institute of Standards and Technology (NIST) is working on developing the quality assurance standards of the additive manufactured products. The current qualification project of NIST is on the methods, metrology and measurements needed for developing a significant understanding of the mechanical performance, qualify 3D printed components and create an efficient post-process measurement [39].
The 3D printing for biomedical applications involves a lot of ethical issues which surpasses freedom to fabricate any biological device with any biomaterial source. However, the therapeutic potential of those biodevices are enormous and, given the novelty of this technology which involves invasive body alterations, 3D printing treatments need ethical challenge management especially with clinical trial stages because there is need to prove its efficiency before application for treatment purposes as the patient, which the fabricated product was used on, is supposed to serve as a sort of guinea pig [41][42]. Therefore, it would be morally reasonable for patient and society safety to be the priority. As a result, this technology should be fully evaluated, articulated and communicated about nature, objectives and risks involved by the manufacturers and clinical professionals.
Although most countries have administrations that cover these issues such as Merger procedure regulation (EC) 139/2004 (European Union), House of Commons Science and Technology Committee Regulation of medical implants in the EU and UK, Food and Drugs Administration (USA), National association for food, drug and administration control (Nigeria), they cover a general application. More fit for purpose regulations should be provided. This might be difficult to execute continuously. A viable framework is crucial to fill in the gap currently. The framework should answer the following parameters: