1000/1000
Hot
Most Recent

Traumatic brain injury (TBI) is defined as an injury caused by an external force that results in the disruption of normal brain function. In the United States, between 2016–2017, there were approximately 451,000 cases of TBI that resulted in hospitalization. The most common mechanisms of injury contributing to TBI were unintentional falls and motor vehicle crashes.
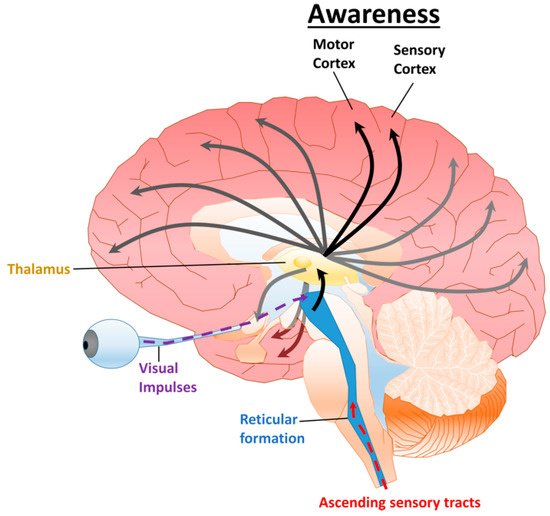
Traumatic brain injury (TBI) is defined as an injury caused by an external force that results in the disruption of normal brain function. In the United States, between 2016–2017, there were approximately 451,000 cases of TBI that resulted in hospitalization. The most common mechanisms of injury contributing to TBI were unintentional falls and motor vehicle crashes [1]. Following a severe TBI, disorders of consciousness (DoC) are common sequela [2][3]. Clinical features correlated with prognosis include age and severity of the TBI [2][4][5][6][7]. In several studies, there is an inverse correlation between the probability of recovering from a DoC and the duration after the injury [5][6][8]; however, some recovery has been observed in patients years after the initial injury [3][9]. The integrity and function of various neural structures and their relationship to consciousness are crucial for predicting outcomes and treating patients [10][11].
| DoC | Arousal | Awareness | Apnea | Eye Opening | Communication |
|---|---|---|---|---|---|
| Brain Death | No | No | Artificial ventilation required | None | None |
| Coma | a No | b No | c Artificial ventilation required | None | None |
| VS/UWS | Yes | No | d Can breathe spontaneously without assistance | Spontaneous | Occasional moans and grunts |
| MCS− | Yes | Partial | d Can breathe spontaneously without assistance | Spontaneous | Occasional facial or vocal activity |
| MCS+ | Yes | Partial | d Can breathe spontaneously without assistance | Spontaneous | Some purposeful facial or vocal responses (inconsistent) |
| Clinical Scoring System | Category | Score Range |
|---|---|---|
| Coma Recovery Scale-Revised (CRS-R) | Auditory Function Scale | 0–4 |
| Visual Function Scale | 0–5 | |
| Motor Function Scale | 0–6 | |
| Oromotor/Verbal Function Scale | 0–3 | |
| Communication Scale | 0–2 | |
| Arousal Scale | 0–3 | |
| Total Score | 0–23 | |
| Glasgow Coma Scale (GCS) | Eye Opening Response | 1–4 |
| Verbal Response | 1–5 | |
| Motor Response | 1–6 | |
| Total Score | 3–15 | |
| Simplified Evaluation of CONsciousness Disorders (SECONDs) | Observation | 0–1 |
| Command-Following | 0–1 | |
| Visual Pursuit | 0–1 | |
| Visual Fixation | 0–1 | |
| Oriented Behaviors | 0–1 | |
| Arousal | 0–1 | |
| * Communication | 0–1 | |
| * Localization of Pain | 0–1 | |
| Total Score | 0–8 | |
| Disability Rating Scale (DRS) | Eye Opening | 0–3 |
| Communication Ability | 0–4 | |
| Motor Response | 0–5 | |
| Feeding (Cognitive Ability Only) | 0–3 | |
| Toileting (Cognitive Ability Only) | 0–3 | |
| Grooming (Cognitive Ability Only) | 0–3 | |
| Level of Functioning (Physical, Mental, Emotional, Social) | 0–5 | |
| Employability | 0–3 | |
| Total Score | 0–29 |
| DoC | CRS-R | SECONDs | DRS |
| Coma | Not Applicable (N/A) | 0 | 29 |
| VS/UWS | N/A | 1 | 22–29 |
| MCS− | Eye Fixation | 2–5 | 17–21 |
| Attention | |||
| Automatic Motor Response | |||
| Localization of Noxious Stimulation | |||
| MCS+ | Consistent Movement to Command | 6–7 | 2–16 |
| Reproducible Movement to Command | |||
| Intelligible Verbalization | |||
| Non-Functional Intentional Communication | |||
| Emerging from MCS | Functional Object Use | 8 | <12 |