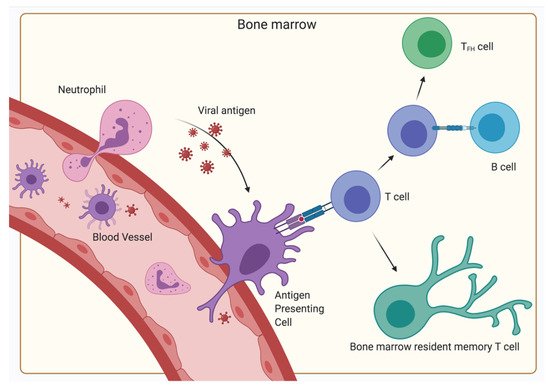
With the BM being such a central organ accommodating a multitude of different kinds of cells, the presentation of antigens and initiation of primary responses—functions typically restricted to SLO—seems to be a possible scenario. Indeed, the initiation of primary T-cell responses of CD4
+ as well as CD8
+ cells to blood-borne antigens have been observed in the BM, indicating an additional function of the BM as a SLO
[25][28]. However, in contrast to classical SLOs, no organized B- and T-cell areas have been described, but instead clusters of dendritic cells and T lymphocytes have been shown
[29][30]. These dendritic cells capture, process and present blood-borne antigen to naïve CD4
+ and CD8
+ T lymphocytes, thereby generating a primary immune response in the BM in the absence of secondary lymphoid organs (Figure 3).
As the BM is not connected to the lymph circulatory system but only the blood circulatory system, the BM might be an important factor for controlling systemic infections. Besides CD11c
+ dendritic cells, neutrophils have also been described as a source of antigen transport to the BM
[31]. Specifically, virus from the dermis is carried to the BM and induces CD8
+ T-cell responses. Along with these primary immune responses, secondary immune responses where memory CD4
+ T-cells are reactivated by antigen have been observed to cause aggregation of immune clusters between MHC II expressing cells and antigen-specific T-cells in the BM
[32]. The MHC II expressing cells were mostly defined as B lymphocytes. This process amplified the T-cell memory and following the termination of the immune reaction, the CD4
+ memory T-cells remained in the BM. These reactions were autonomous to the BM, ergo independent of immigrating T-cells. Even though B-cells were involved, no humoral memory adaptation or GC formation was detected. Furthermore, the expression of signature genes of follicular helper T-cells was significantly lower than in the spleen, indicating a non-follicular reactivation. However, there is some evidence, that dendritic cells may activate CD4
+ T-cells and license them to differentiate into resting memory cells in the BM during primary immune responses, while some activated CD4
+ T-cells interact with bystander B-cells as a follow up to the initial antigen presentation, leading to their differentiation into T
FH (Figure 3)
[33]. Furthermore, some studies suggest that BM memory CD4
+ T-cells can differentiate into T
FH cells during a recall response, indicating that some are committed to the T follicular helper lineage
[34]. T
FH cells are important for many processes typically associated with SLOs. Memory T
FH cells are most likely sustained by a persistence of antigens, potentially via CD11c
+ or B-cell presentation
[35]. On the other hand, BM resting memory CD4
+ T-cells are typically independent from antigen signals; hence, the ratio of T
FH cells and BM resting memory cells might be affected by antigen persistence. Interestingly, while T
FH cells play an important role in promoting plasma cell survival in SLO via production of IL-21, the plasma cell maintenance in the BM is independent of T
FH support as BM plasma cells do not express IL-21R
[36]. Overall, while the BM has some competences of a SLO, it is not capable of fulfilling the complete role of a SLO. However, it is unique in the sheer amount of functions it has to implement, being capable of performing primary and secondary immune functions and hemato- and lymphopoiesis. Particularly, the systemic immune control of blood-borne antigen heavily relies on the BM.