1000/1000
Hot
Most Recent

This entry summarizes molecular mechanisms of intracellular membrane traffic, mainly focusing on the secretory and endocytic pathways, in A. oryzae and its related filamentous fungi.
The reason why A. oryzae cells can abundantly produce valuable materials was not fully understood with respect to molecular mechanisms of intracellular membrane traffic [5]. Since the completion of A. oryzae genome analysis in 2005, reverse genetic approaches have been widely applied to understand molecular mechanisms of both the secretory and endocytic pathways in the fungus [1,6]. Especially, fluorescent protein-based cellular biological analysis has greatly advanced the understanding of molecular machinery, especially about the predominant apical secretion [1,5]. This review summarizes molecular mechanisms of intracellular membrane traffic, mainly focusing on the secretory and endocytic pathways, in A. oryzae and its related filamentous fungi.
A hallmark of secretory proteins produced by A. oryzae is α-amylase, encoded by three almost identical genes amyA/B/C, with respect to the production quantity [9]. Historically, α-amylase, also known as Taka-amylase A from Takadiastase, was isolated and crystallized from A. oryzae cultures in the 1950s [10,11]. Indeed, localization analysis by an indirect fluorescent-antibody technique using the antiserum against α-amylase demonstrated that α-amylase is located on the cell surface [13]. About a half-century later, recent analyses revealed that a cell wall component α-1,3-glucan is a potential inhibiting factor for α-amylase adsorption onto cell walls in the A. oryzae submerged culture [14].
A. oryzae also secretes other carbohydrate hydrolases than α-amylase, including glucoamylase and α-glucosidase [2,9]. characterized in A. oryzae: There are 135 secretory protease genes predicted by the presence of signal peptide in the A. oryzae genome, among which pepA is a well-analyzed gene encoding acid protease [6,21]. The disruption of pepA enhanced the secretory production of heterologous proteins by avoiding degradation of the secreted proteins [22].
Generally in A. oryzae, secretory proteins are more produced in solid-state culture (SSC) than in submerged culture [24]. In addition, there are certain proteins that are secreted specifically in SSC, but not in submerged culture; for example, a glucoamylase-encoding glaB is expressed and its protein is secreted only in SSC [25,26]. Moreover, as examples of industrial SSC, proteomic analyses on soy sauce fermentation using soybeans and wheat as the culture substrates identified extracellular proteases and amylolytic enzymes responsible for the generation of soy sauce flavors Due to stable interaction between A. oryzae and A. niger cells, this co-cultivation would have the potential for engineering enzyme cocktails.
Conventional secretory proteins harboring a signal peptide at the N-terminus are initially targeted to the endoplasmic reticulum (ER). Through ER and Golgi, most of the secretory proteins are modified with N-glycans. Although the molecular mechanisms of both N- and O-glycans have been relatively well investigated in filamentous fungi, especially N-glycosylation mechanisms related to secretory proteins have been analyzed in A. oryzae. For the secreted N-GlcNAc-proteins, the remaining single GlcNAc moiety onto the glycoprotein might be important to maintain the protein structure and function.
To dissect the secretory machinery in A. oryzae, enhanced green fluorescent protein (EGFP)-tagged α-amylase Moreover, both AmyB-EGFP and RntA-EGFP are also observed at septa, suggesting that there is molecular machinery for septum-directed secretion (Figure 1B) [44]. Indeed, fluorescence recovery after photobleaching (FRAP) analysis demonstrated that there is a constant flow of AmyB-EGFP to septa. Furthermore, secretion of AmyB-EGFP and RntA-EGFP to the hyphal tip is dependent on actin and microtubule cytoskeletons; in contrast, that of AmyB-EGFP to the septum is dependent on microtubule but independent of actin, suggesting that there are different molecular mechanisms between secretion to the hyphal tip and that to the septum [44].
In other filamentous fungi, there is a possibility that lateral secretion through the entire plasma membrane, not restricted from the tip and septum, might occur [45]. In fact, for A. oryzae cells, a recent report demonstrated that transient physical plasma treatment induces depolarization of the plasma membrane and activation of calcium ion influx into cells, resulting in increased α-amylase secretion [46]. Besides extracellularly secreted proteins, cell-wall-forming enzymes that are transported to the plasma membrane via vesicular trafficking have been well investigated [47]. In a model fungus Ustilago maydis, chitin synthases and 1,3-β-glucan synthase are transported in the same vesicle to the plasma membrane, suggesting that cell wall is synthesized locally by these enzymes, although whether such a transport mechanism exists in A. oryzae needs to be examined [48].
To understand the intracellular dynamics of secretory proteins, the subcellular localization of ER is crucial because the organelle is the initial part of the secretory pathway. This localization pattern of ER suggests efficient protein secretion mainly from the hyphal tip. The deletion of Aovip36 or Aoemp47 improved heterologous protein secretion, suggesting that AoVip36 and AoEmp47 retain secretory proteins in ER and Golgi [50]. Furthermore, a genome-scale analysis suggested that an A. oryzae ortholog of Saccharomyces cerevisiae Erd2p that functions in the retrieval of ER-resident proteins from Golgi is involved in essential secretion machinery [51].
In the intracellular vesicular trafficking, vesicles need to be properly transported to the target membrane, in which soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) have important roles [52]. One vesicule-SNARE (v-SNARE) and three target-SNAREs (t-SNAREs) make a complex to allow the membrane fusion of a vesicle and the target membrane. Based on the genome information of the model yeast S. cerevisiae, reverse genetic analyses identified 21 SNAREs that existed in A. oryzae[53]. Comprehensive localization analysis of EGFP-fused SNAREs revealed that most of the proteins predictably reside at each membrane compartment; for instance, a v-SNARE AoSnc1 exhibits motility and localizes to secretory vesicles from Golgi to the plasma membrane and mainly at Spitzenkörper.
A. oryzaeα-amylase is not only abundantly secreted, but also α-amylase genes are highly transcribed, and the regulatory mechanisms of α-amylase genes have been well investigated [9]. It is also known that maltose is an inducing factor for the gene expression of α-amylase as well as other starch-degrading enzymes. Although biochemical mRNA expression analysis has been widely conducted and the molecular mechanism for α-amylase secretion has been well investigated as described above, little is known about the subcellular location of transcription and translation of α-amylase.
In A. oryzae cells, single-molecule fluorescence in situ hybridization (smFISH) was recently conducted [54]. smFISH is one of the methods for mRNA localization analysis by using multiple fluorescent probes, which enable visualization of a single mRNA molecule [55]. Moreover, induced expression of α-amylase mRNAs with maltose addition was observed throughout the hyphal cells, suggesting the presence of α-amylase secretion not only at apical and septum but also at basal regions [54]. Since the nucleus localized closest to the apex is generally more than 10 µm away from the tip, actA mRNAs might be actively transported from the nucleus to the hyphal tip where actin proteins are also localized [54].
Although most of the proteins with signal peptides are thought to be secreted from the hyphal tip in filamentous fungi, there exist certain proteins lacking signal peptides that undergo unconventional protein secretion (UPS) [ Although there are only a few reports of UPS proteins available in filamentous fungi, a chitinase Cts1 of U. maydis is known to be unconventionally secreted in a lock-type manner [61]. In addition, Cts1 was successfully employed as a UPS carrier protein for heterologous protein production without modification of glycosylation onto the secreted heterologous proteins [62,63]. This provides further evidence that Cts1 bypasses ER and Golgi where glycosylation occurs.
In A. oryzae, an acyl-CoA binding protein AoAcb2, one of S. cerevisiae Acb1 orthologs, was characterized as a UPS protein (Figure 1B) [64]. AoAcb2 that lacks signal peptide was found to be secreted under carbon starved conditions, but not under nitrogen starved conditions. Moreover, the UPS of AoAcb2 is dependent on the presence of the plasma membrane t-SNARE AoSso1, suggesting that AoAcb2 is secreted via vesicular trafficking. These UPS properties of AoAcb2 are similar to those of a peptidase PepN in A. niger[65].
A recent analysis in A. nidulans reported that a model purine transporter UapA is transported from ER to the plasma membrane via Golgi bypass [66,67]. Investigation of neosynthesized UapA revealed this UPS pathway that is dependent on COPII vesicles, actin polymerization, clathrin heavy chain and the plasma membrane t-SNARE SsoA. Importantly, this UPS pathway of UapA is also applied to translocation of AzgA and FurA, purine and allantoin transporters, respectively. Whether such a UPS pathway for plasma membrane transporters, including AoUapC and AoGap1 that are known to be transported to the septum, exists in A. oryzae needs to be elucidated [44]
Recently, not only proteins but also certain metabolites were found to be secreted via intracellular membrane trafficking in filamentous fungi [68,69]. For example, A. oryzae extracellularly produces kojic acid (KA) as a secondary metabolite, which is used as a skin-lightening agent in cosmetics (Figure 1B) Although the KA biosynthetic processes are less understood, so far four KA biosynthesis-related genes—namely kojA, kojR, kojT and kpeA—were identified [71,72]. KojR and KpeA are Zn(II)2Cys6transcriptional activator and repressor, respectively, that are thought to regulate the expression of kojA and kojT genes, which putatively encode an enzyme and a transporter, respectively.
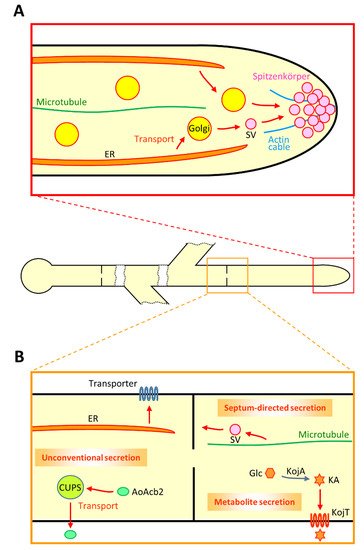
Although A. oryzae does not produce citric acid extracellularly, the black Koji mold A. luchuensis and its albino mutant A. kawachii can secrete plenty of citric acid [78,79]. Especially in A. kawachii, molecular mechanisms of citric acid secretion have been well investigated [80]. CtpA and YhmA are transporters localized to the mitochondrial membrane that transport citric acid from mitochondria to the cytoplasm [81]. CexA is a plasma membrane transporter responsible for the extracellular secretion of citric acid from the cytoplasm, and the transcription of cexA is regulated by LaeA [82].
Endocytosis is one of the conserved cellular processes that occur at the plasma membrane of eukaryotes for the acquisition of extracellular nutrients, internalization of plasma membrane proteins and reconstruction of cell polarity [84]. Most of the filamentous fungal genomes harbor homolog genes of endocytic proteins that were already identified in other eukaryotic cells [85]. However, in filamentous fungi, molecular mechanisms and physiological roles of endocytosis were not examined well. As one of the initial applications for the investigation of endocytosis in filamentous fungi, the lipophilic dye FM4-64 was employed, which was generally used as an endocytic marker in yeast S. cerevisiae[86,87].
Indeed, the repression ofAoend4resulted in defects of severe growth and endocytosis, analyzed by using FM4-64 and AoUapC-EGFP. However, inAoend4-repressed hyphae, EGFP-AoSnc1 was mislocalized to the whole plasma membrane, likely due to the lack of endocytosis. These results suggest that endocytosis is crucial for apical growth and recycling of certain components required for vesicular trafficking [89]. Furthermore, transmission electron microscopy revealed that cell wall components were accumulated at large invaginated plasma membrane structures in endocytosis-deficient hyphae, suggesting that cell wall synthases also undergo endocytic recycling.
Based on the investigations of the endocytosis-deficient mutant in A. oryzae, a model of endocytic recycling at the tip region was proposed (Figure 2A). A. oryzae can secrete large amounts of proteins, such as α-amylase, to the medium and this apical endocytic recycling mechanism may support such enormous secretion capacity. The endocytic recycling model has been widely accepted in filamentous fungi, and other recycling proteins have been identified [47,91,92,93]. Collectively, apical endocytic recycling is closely connected with continuous growth and secretion at the hyphal tip region.
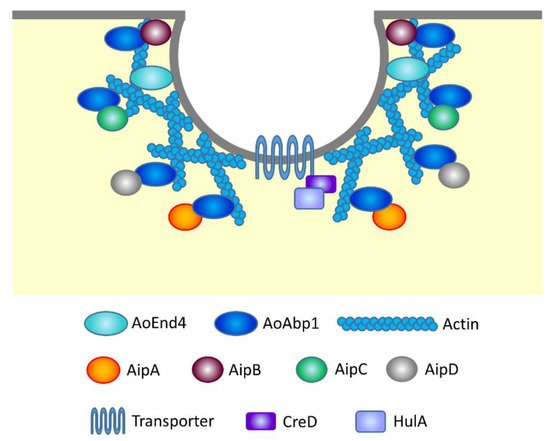
It is well known that actin and its related proteins are involved in endocytosis by forming actin patches that generate force to pull the plasma membrane into the cell’s interior [94]. possesses one SH3 domain that functions in protein interaction, whereas AoAbp1 harbors two SH3 domains. This difference raised the possibility that there exist discrete protein interactions with AoAbp1, which are not found in S. Therefore, yeast two-hybrid screening using AoAbp1 as bait and A. oryzae total library proteins as prey was conducted, and four
A maltose permease MalP localized at the plasma membrane has been analyzed as a protein that undergoes endocytosis in A. oryzae[97]. In the presence of maltose in the medium, MalP at the plasma membrane incorporates maltose to induce the expression of amylolytic enzyme genes. In contrast, in the presence of glucose, MalP is endocytosed and the expression of amylolytic enzyme genes and malP is repressed. Whether endocytosis of other plasma membrane transporters, such as AoUapC and AoCan1, is also dependent on HulA and/or CreD needs further investigations (Figure 3).
Endocytosed proteins are first transported to early endosome (EE), then to late endosome (LE) and In mammalian cells, EEs are maturated to LEs by conversion of Rab5 to Rab7, the molecular mechanisms of which are thought to be conserved in filamentous fungi [100,101]. In filamentous fungi, Rab5-positive EEs are highly motile and move through the cell; by contrast, Rab7-positive LEs are mostly static and in general observed adjacent to vacuoles [99,101,102]. EEs motility is a hallmark of endocytic organelles in filamentous fungi and
To properly transport certain proteins, including proteases, into vacuoles, there is the molecular machinery of vacuolar protein sorting (Vps). In S. cerevisiae, mutants of VPS genes were identified by the screening using carboxypeptidase Y (CPY) as a Vps cargo, in which CPY was not transported to vacuoles but missorted to the medium [103,104,105]. Accordingly in A. oryzae, VPS mutants were visually isolated by using CPY-EGFP [106]. The deletion of Aovps24 resulted in defects of proper vacuolar formation and mycelial growth, and the same phenotypes were observed in the deletion of another ESCRT-III gene Aovps2, suggesting that ESCRT-III components are required for vacuolar formation that is essential for mycelial growth [108].
In A. oryzae hyphal cells, EE dynamics were firstly visualized with AoUapC-EGFP after the induction of endocytosis by the addition of ammonium to the medium [88]. The detailed molecular machinery underlying the constant long-range EE motility has not yet been characterized in A. oryzae, but well understood especially in other model filamentous fungi U. maydis and A. nidulans[109,110,111]. EEs move bidirectionally along MTs by motor proteins kinesin-3 and dynein towards MT plus and minus ends, respectively [112,113,114,115,116]. In the mutants of Hook, EEs become immotile but motor proteins still move [117].
This observation raised the possibility that EEs have other physiological roles than endocytic function. Indeed, investigations in U. maydis revealed that constant EE motility distributes translationally active polysomes and other organelles, such as ER, peroxisome and lipid droplet in hyphal cells [102,120]. To elucidate further physiological roles of EE motility, the deletion mutant ofAohok1, the Hook ortholog in A. oryzae, was investigated [99]. Taken together, EE motility has various physiological functions in filamentous fungi and further investigations are required to elucidate whether EE motility is involved in signal transduction in A. oryzae.
The vacuole is an acidic organelle and has crucial physiological roles, such as storage of metabolites and regulation of cytoplasmic homeostasis [123]. Visualization of vacuoles in A. oryzae cells was initially conducted with CPY-EGFP that localizes in the vacuolar lumen and demonstrated pleiomorphic vacuolar structures [124]. However, since fluorescence of CPY-EGFP in vacuolar lumen varied depending on culture pH, EGFP-fused t-SNARE AoVam3 that localized to the vacuolar membrane was employed to stably visualize vacuoles [124,125]. Indeed, visualization of EGFP-AoVam3 revealed that vacuoles are highly dynamic, some of which exhibit not only spherical and cubic structures but also moving small punctate and tubular structures [125].
Autophagy is a key physiological event that occurs in vacuoles under nutrient starvation conditions to maintain cellular homeostasis [127]. A. oryzae is one of the model filamentous fungi to study autophagy machinery. Moreover, autophagy might be involved in supporting nutrient transport from vacuoles of basal hyphal regions via tubular vacuoles to aerial hyphal cells where nutrients are not supplied from the cell exterior through the plasma membrane [130,131]. Moreover, related to autophagy machinery, an acyl-CoA binding protein AoAcb1 exhibits long-range motility in the cytoplasm and its subcellular localization is regulated by autophagy proteins; however, the physiological importance of the regulation on AoAcb1 localization remains yet unknown [134].
Due to the great ability of valuable enzyme secretion, the molecular mechanisms of the secretory pathway were investigated in A. oryzae[1]. Moreover, it was shown that under stress conditions, stress granules that consist of non-translating messenger ribonucleoproteins are formed around the hyphal tip region of A. oryzae cells [138], although the underlying molecular and physiological details of stress granule formation need further analyses. Furthermore, novel findings on membrane traffic related to SM biosynthesis would provide a new strategy for improving valuable SM production Finally, because genome editing was successfully applied to A. oryzae[141,142], molecular manipulation based on discoveries in membrane traffic would be favorable, especially onto industrial strains for further valuable material production.