1000/1000
Hot
Most Recent

Social egg freezing (SEF) allows women to preserve their fertility in anticipation of age-related fertility decline and ineffective fertility treatments at older ages. Social freezing is the term used when eggs or ovarian tissue are frozen for professional, personal, financial, and/or psychological reasons and used later in life.
The fertility preservation field has developed over the last two decades, but data regarding its results are limited. An increasing number of women choose to delay the conception of a child for various social reasons. The most common cause of delaying childbearing cited by women is the lack of a partner suitable for creating a family. The second reason is professional, related to completing education, career advancement, and inflexibility in the workplace, with women considering becoming pregnant before 35 years as affecting their career [1][2][3].
Women who choose elective egg freezing are commonly Caucasian, between 36 and 40 years of age, with higher education, professional employment, and without a partner [1][2]. (figure 1)
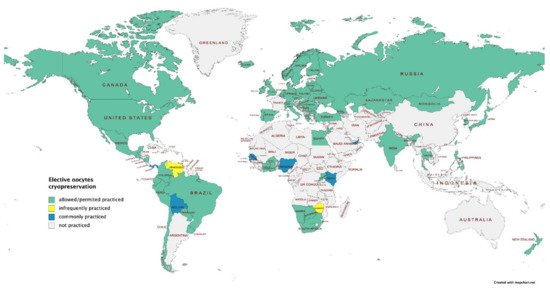
Figure 1. Elective oocytes cryopreservation: worldwide map (data provided by International Federation of Fertility Societies’ Surveillance [4], created with Mapchart.net).
Women who choose elective oocyte cryopreservation may address their gynecologist or fertility preservation team, consisting of an embryologist, fertility specialist, and a psychologist or counselor [5]. However, for quality decision-making, they should be informed of the risks of the procedure, benefits and costs, the success rates, long-term outcomes regarding physical health, psychological well-being, currently known data on the health of children born from frozen oocytes, duration of storage of frozen eggs, and sign an informed consent form [6][7][8].
The purpose of our study was to systematically review the recent findings focusing on the elective egg freezing challenges published in the last decade in primary databases.
We performed a systematic qualitative review in the present study according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. For this purpose, we performed a preliminary search on Google Scholar. We systematically searched PubMed®/MEDLINE (http://www.ncbi.nlm.nih.gov/ PubMed, accessed on 15.01.2022 ) and Web of Science® databases for recent literature as systematic reviews (SRs), meta-analyses (MAs), (randomized) clinical trials (RCT/CT), and clinical case series (CCS) related to elective egg freezing.
Thirty-seven studies were included in the present review, centered on the five main topics included in the search. More detailed information regarding the selection process is presented in the PRISMA flow diagram (figure 2).
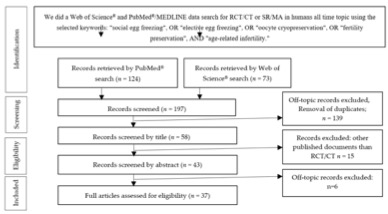
Figure 2. Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) diagram describing our systematic search and study selection process (RCT, randomized clinical trial; CT, clinical trial).
Fertility can be preserved for medical or non-medical reasons with similar success rates [9] through oocyte, embryos, or ovarian tissue cryopreservation. Cryopreservation involves storing cells and tissues at negative temperatures for extended periods, using cryoprotective additives to prevent ice formation. Oocyte vitrification is the technique of choice for oocyte cryoprecipitation [10]. Using the Cryotop protocol employed for oocyte vitrification initially established by Kuwayama et al. in 2005 [11].
Oocyte cryopreservation is an elective method for fertility preservation for age-related infertility and involves ovarian hormonal stimulation, oocyte retrieval, freezing, and oocyte storage [2][12]. As of 2013, the American Society for Reproductive Medicine (ASRM) no longer considered oocyte cryopreservation an experimental procedure to become a realistic option for fertility preservation against age-related decline [2]. In 2016, Argyle et al. conducted a systematic search on the topic, reporting a rise in the success rate of oocyte preservation and increased use of the vitrification technique, generating in vitro fertilisation (IVF) pregnancy rates similar to those achieved using fresh oocytes [10].
Embryo cryopreservation is widely available in fertility preservation but needs legal ownership between both partners, leading to further difficulties. For example, a woman who separates from their partner with the embryos may not become pregnant if they retract their consent [6].
Freezing of ovarian tissue is another method used for medical or non-medical fertility preservation. For example, it allows retrieval of endocrine function, offers the possibility of natural conception after tissue transplantation, and postpones menopausal symptoms and related conditions [2][13].
The optimal timing for a woman to freeze her eggs, with a much higher success rate, is under 35 years, as after this age, the quality and number of eggs decline [6][14]. Mesen et al. found maximum benefits when cryopreservation is performed between 32 and 37 years, with little benefit at ages 25–30 [15]. Cryopreserving eggs at an earlier age can minimize the number of cycles necessary to obtain sufficient eggs and maximize egg quality, with the risk of never using them. With increasing age in women at the time of oocyte cryopreservation, outcomes are more negative. Women who are older at the time of ovarian stimulation for oocyte preservation require higher gonadotropin doses and storage of more oocytes of lower quality [16][17]. Cryopreserved eggs can be fertilized and implanted until the age of 54 years in Israel.
The number of oocytes retrieved should be individualized depending on the patient’s age, clinical circumstances, and ovarian reserve. Currently, special programs are available that help us calculate the number of oocytes needed to be harvested depending on the patient’s age to increase the risk of getting pregnant and to predict the live birth rate probability [18]. Women can attempt a maximum of four oocyte retrieval cycles; the ideal number of retrieved oocytes is 20 [7][17]. Storage of ten oocytes produces a probability of live birth per thawed oocyte of 60.5% in women under 35 years, compared to 29.7% in women aged over 35 years [19]. Cobo et al. [19] found that pregnancy rates are age-dependent and estimated that a minimum of 8–10 oocytes are necessary to achieve pregnancy, whereas Doyle et al. revealed that the use of 20 oocytes results in a CLBR of 60–80% in women between 35 and 38 years old [16]. A total of 15 to 20 oocytes retrieved in freeze-all cycles ensures a balance between the procedure’s safety and efficacy with individualized stimulation protocols [20].
Artificial intelligence (AI) was recently used to assess the quality of embryos and oocytes to create a highly predictable model for ART, which was able to recognize and classify embryos and oocytes [21] automatically. The development of AI has allowed the integration of new technologies in ART automation by processing oocytes (denudation by removing cumulus cells and oocytes positioning) to improve the efficiency and quality of ART [22].
The application of OMICS platform technologies in the field of egg freezing was found to be a challenging method of assessing the quality of oocytes [23][24] (figure 3)

Figure 3. The OMICS technologies used for oocytes assessment.
Recent studies demonstrated the safety of using vitrified oocytes on neonatal outcomes and genetic diseases, with the risk of congenital anomalies being the same as natural conception or following IVF treatment with fresh oocytes. No risks specific to freezing are known [25][26][27]. The age-associated risk of fetal loss and aneuploidy can be reduced using younger oocytes [27]. Medical risks associated with cryopreservation surround those surrounding ovarian stimulation and oocyte retrieval [1][2][12]. We also consider the risks associated in older women who achieved pregnancy using vitrified oocytes or IVF - a high risk of ectopic pregnancy, first-trimester losses, and obstetric risks such as preeclampsia, gestational diabetes, preterm birth, low birth weight, and cesarean birth [1][2][28].
Regarding the usage rate, the percentage of women who return to use their frozen oocyte, Cobo et al. found that 9.3% of women used their oocytes at 39.2 years old, 2.1 years after freezing time, on average [19]. The reasons for the low usage rate of frozen oocytes were found to be single-parent issues, the preference to conceive naturally, and not wanting to use a sperm donor [29], as well as insufficient knowledge and interest of women in the procedure at the optimal age range to perform FP (28–35 years). Frozen oocytes can be stored for unlimited time without deterioration. However, legislation from the U.K. allows storage for a maximum of 10 years; after that, eggs must be discarded, limiting the success rate [2].
Decision regret is an indicator of the quality of health decisions, measured by the decision-regret scale (DRS), consisting of negative emotions following the decision. Decision regret was absent in 51% of women who electively cryopreserved their oocytes (from 2012 to 2016), while 33% reported mild decision regret, and 16% experienced moderate-severe decision regret. Factors associated with increased decision regret were a low number of frozen oocytes, a poor understanding of information and emotional support during the freezing process, and a reduced estimated probability of achieving a live birth using cryopreserved oocytes [30].
However, various ethical considerations surround elective fertility preservation. The Ethics Committee of the American Society of Reproductive Medicine found that planned oocyte cryopreservation ethically allows, increasing women’s reproductive autonomy and promotes social equality [31]. Although it is still debated in several countries (Austria and France), and it is recommended (Israel) [32][33][34]. The ethical debate of the topic arouses many issues such as commercial exploitation, the medicalization of reproduction, women’s autonomy, idealization about the right time for a pregnancy, the impact of egg freezing on sex inequality, and professional norms [2][35][36].
Oocyte cryopreservation for age-related fertility loss is provided in the private sector, so costs are borne by the patients; some patients with private medical insurance may have part of the costs covered [7]. When estimating cost-effectiveness (procedure-related benefits) women must consider the usage rate of the oocytes [1][10][37]. Elective egg freezing at a younger age can reduce the costs associated with infertility treatment and would be more successful and cost-effective [36] if performed at 35 [15] or 37 years of age [38]. An important cost consideration to discuss is the number of desired children; as the number of desired children increases, the procedure may be more cost-effective at a younger age [36]. Loendersloot et al. demonstrated that the oocyte-freezing procedure is cost-effective if 61% of the women return to use their frozen eggs later in life [38].
In this study, we only approached the female side of gamete conservation. The social freezing of sperm is different from the social freezing of oocytes, being less focused on preserving fertility, instead of reducing increased genetic risks due to advanced paternal age. Sperm freezing is most often recommended for male cancer patients, those with vasectomy, or those exposed to toxic environmental factors [39].
The future is represented by the involvement of nanotechnology, microfluidic biophysics, and oocyte culture using immature eggs from fresh ovarian tissue to establish the effectiveness of complete in vitro growth (IVG) of follicles, in vitro maturation (IVM), and in vitro fertilization technology (IVF) [40].
Fertility preservation clinicians should properly inform women about the safety, efficacy, benefits, usage rate, risks, and costs of the procedure and offer them real expectations to mitigate potential harms. Due to the novelty of the procedure, many women are less aware of the safety and efficacy of the procedure, including the possible complications of a pregnancy at an older age. Future research on usage rate, live birth rate, pregnancy outcomes, and long-term follow-up of children conceived using frozen-thawed oocytes are necessary.
The entry is from 10.3390/ijerph18158088