3.1. Role of Selenium in Steroidogenesis and Spermatogenesis
The optimal concentrations of primary sex hormone (testosterone) are essential for normal development of sperm cells. During the process of testosterone biosynthesis, ROS are generated and their excessive production contributes to male infertility
[47]. Interestingly, almost two decades ago,
Selenop mRNA was selectively identified in Leydig cells of rats
[47][48]. It was suggested that, in addition to its role as a plasma selenoprotein transporting Se, Selenop may also play a role as an intracellular antioxidant in Leydig cells
[48]. Besides, cytosolic Gpx was also implicated in counteracting the H
2O
2 generated as a result of testosterone biosynthesis, but its expression in testis was relatively lower
[47]. However, as for the effects of Selenop on steroid biosynthesis in Leydig cells, it was proposed that, in addition to its intracellular antioxidant role, Selenop might also act as an extracellular antioxidant protecting Leydig cells from oxidative damage. Subsequently, in 2001, using the mouse and rat models, a physiological function of Selenop in testosterone production was reported in Leydig cells
[47]. It was suggested that Selenop plays an important antioxidant role in protecting Leydig cells from the oxidative damage resulting from testosterone biosynthesis pathway
[47][48].
In any case, the growing body of literature also suggests that Se is also important for the biosynthesis of testosterone. It has been suggested that blood testosterone concentrations have positive correlation with concentrations of Se
[49][50]. Similarly, dietary Se supplementation has been demonstrated to ameliorate the testosterone level and quality of semen in different animal species
[12][51][52][53]. However, the exact underlying mechanisms regulating the testosterone production are yet unclear and require further understanding. Since testosterone is produced by Leydig cells, they could serve as a potential target model to further investigate such mechanisms of Se-related modulation of testosterone synthesis
[54]. It has been demonstrated that Se has a potential regulatory role in some essential cellular functions. This role is reportedly modulated by activating the extracellular-signal-regulated kinase (ERK) signaling pathways
[55]. However, the exact role of Se in biosynthesis of testosterone via modulation of ERK signaling pathway and the fate of Leydig cells throughout the process of sperm development is incompletely understood
[54]. In this connection, in an in vitro study, Shi et al.
[54] revealed that Se could modulate the proliferative and apoptosis-related cellular events in ovine Leydig cells. These effects were principally achieved via regulation of oxidative stress, cell cycle, and apoptosis-associated biomarkers. The lowest ROS content and the highest GPX activity were found in the 2.0 μmol/L group compared to the control (without Se) and high Se-treated (4.0 and 8.0 μmol/L) groups. They further reported that Se could also increase testosterone biosynthesis in Leydig cells via activation of ERK signaling pathway and expression of its downstream genes i.e., steroidogenic acute regulatory (
StAR) and 3 beta-hydroxysteroid dehydrogenase (
3β-HSD). These biomarkers are thought to have a close relation with the regulatory implications of Se in fertility and gametogenesis in males. Optimal levels of Se (2.0 μmol/L) triggered a significant up-regulation in the expression of these genes and improved testosterone biosynthesis. However, when the concentration of Se was increased (8.0 μmol/L) in culture medium, the proliferative capacity of Leydig cells and the expression of cell cycle-related biomarkers were reduced, and the ratio of cells undergoing apoptosis was also significantly raised. Intriguingly, latter findings were coherent with the relative expression of pro-apoptosis-related markers
[54]. Interestingly, maternal dietary Se supplementation (0.5, 2.0 mg Se/kg dry matter (DM)) led to the statistically higher indices related to testes histomorphology, and significantly higher (compared to the controls) density of spermatogenic cell lines and Leydig cells was recorded in testicular parenchyma of young “Taihang Black” male goats
[56]. In contrast, these indices (testicular weight and volume) were significantly decreased in Se-excess (4.0 mg/kg) group. Improved levels of testosterone in testicular tissue and serum, and increased expression of testosterone biosynthesis-related biomarkers were observed in Se-treated groups. The relative mRNA expression of
StAR, 3β-HSD and cytochrome P450 family 11 subfamily A member 1 (
CYP11A1) was reduced when the level of Se was increased in the maternal diet. Supplementation of Se in maternal diet could also influence the expression of androgenic receptor protein in testes of resulting progeny
[56]. Furthermore, in an interesting comparative transcriptomic study on testes of new born bovine calves, Cerny and colleagues
[57] reported that the form of Se (35 ppm organic Se, 35 ppm inorganic Se, and 50:50 mix of both (MIX)) fed to cows during gestation differentially impacted the expression of several mRNAs putatively regulating the encoding proteins implicated in steroidogenesis and/or spermatogenesis. Relatively pronounced and desirable transcriptional expression of mRNAs implicated in steroidogenesis and spermatogenesis were observed compared to the calves born to cows fed inorganic Se during gestation
[57]. Of note, testes of neonatal calves born to cows fed MIX (50:50 organic and inorganic Se) showed an independent transcriptomic phenotype that was not intermediate between the organic and inorganic groups
[57]. It is worthwhile to mention that all claves used in this analysis were of high/adequate Se status and were born to the dams of high/adequate Se status. However, at present, the mechanistic and physiological bases of these differential transcriptomic events are incompletely understood and future well- powered studies with adequate number of animals are awaited. Nevertheless, previously it has been demonstrated that organic Se resulted in the higher blood and liver tissue levels of Se compared to the inorganic supplemented cows
[57][58][59].
Similar to its role in steroidogenesis, Se has also been implicated to play an important role in spermatogenesis. In one of the classical studies on the role Se in male fertility and reproduction, Watanabe and Endo
[60] examined the morphology of sperm and spermatocyte chromosomes in mice fed Se-deficient diet. They reported that the ratio of abnormal sperm was high (6.8% to 49.6%) in Se-deficient group compared to the control group (4.0% to 15.0%). The morphological defects were more pronounced in sperm head compared to other regions i.e., midpiece and tail. However, the frequency of chromosomal abnormalities in spermatocytes (MI stage) was comparable between Se-insufficient and the control groups
[60]. Besides, in another study, Se-deficient (yeast-based Se 0.02 ppm) feed significantly reduced the number of spermatogenic cell line i.e., pachytene spermatocytes, spermatids and maturing sperm in mice
[61]. Taken together, these findings corroborate that Se might have strong implication in the process of spermatogenesis in males.
Recently, in an in vitro cell culture study on Sertoli cells of newborn bovine calves, it has been shown that Se supplementation (inorganic Se; 0.25, 0.50, 0.75, and 1.00 mg/L) in culture media could influence the cell viability and expression of essential protein components (occludin, connexin-43, zonula occluden, E-cadherin) of blood–testis-barrier. In fact, this effect of Se was dose-dependent and exhibited the U-shaped response i.e., optimal ameliorative effects were observed at moderate doses (0.25, 0.50), and temporary cytotoxic effects were evident when the dose was increased to 0.75, and 1.00 mg/L
[62]. Interestingly, the mechanistic basis for these effects of Se was later elucidated by the same group of researchers
[63], where they demonstrated that Se-pretreatment could ameliorate microcystin-LR (MC-LR)-triggered cytotoxicity in bovine Sertoli cells. Pre-treatment with inorganic Se (0.50 mg/L) inhibited the nuclear factor kappa B (NFκB) activation in cultured Sertoli cells. Selenium also exhibited some immunomodulatory roles, which were evident by the inhibition of inflammatory cytokine activation in MC-LR-exposed Sertoli cells
[63]. Besides, Se-pretreatment also modulated the expression of mitochondria-related genes such as Cytochrome c oxidase subunit I (
COX-1), Cytochrome c oxidase subunit 2
(COX-2), Acetyl-CoA acetyltransferase 1
(ACAT1), Mitochondrial transcription factor A (
mtTFA) and Subunit 2 of NADH dehydrogenase (
MT-ND2). Se-treatment also modulated the apoptotic events in Sertoli cells, which were highlighted by the inhibitory effects on apoptosis induction via cytochrome-c release and expression of caspase-3. Mitophagy and mislocalization of components of blood–testis-barrier were also inhibited in Se-treated Sertoli cells compared to the MC-LR-exposed group. Intriguingly, the peculiar antioxidant effects of Se were also observed, which were evident by an increased GPX4 activity in cultured Sertoli cells
[63]. Since, NFκB and mitochondrial signaling pathways, and blood–testis barrier (an essential measure for protecting the testicular parenchyma) are implicated in delicate cellular functions in testicular parenchyma, results of these studies reasonably provide the novel and enticing evidence that Se, at least in part, could help ameliorate the Sertoli cells-related perturbations is spermatogenesis and male fertility. Nevertheless, both in vitro and in vivo studies involving other animal models will be interesting, and have the potential to provide concreate evidence with regards to ameliorative roles of Se in Sertoli cell function and spermatogenesis.
There is also an evidence (in vivo) that Se might have an implication in modulating the process of spermatogenesis via regulation of expression of genes related to cell cycle and apoptosis
[64][65][66]; however, the precise mechanistic basis for this Se-mediated modulation is still unclear
[54]. It has been demonstrated that components (
cjun and
cfos) of redox active transcription factor, Activator protein 1 (AP-1), modulate the proliferation and differentiation of cells, and they are also implicated in modulating the signal transduction pathway, and also play a regulatory role in steroidogenesis and spermatogenesis. It has also been shown that the expression of transcription factor (AP-1),
cfos and
cjun, is stage specific and increased during the phase of active spermatogenesis
[67]. It was reported that when mice were fed a Se-deficient diet (yeast-based diet with 0.02 ppm Se), a significant decline in testis Se-levels was observed compared to those who received a Se-adequate (0.2 ppm inorganic Se) diet, and a significant increase was observed in Se-excess group (1 ppm in organic Se) compared to the Se-adequate and Se-deficient groups. GPX activity and lipid peroxidation (LPO) levels were also decreased and increased, respectively in Se-deficient group. Interestingly, a significant increase was also observed in the mRNA and protein expression of cfos and cjun in Se-deficient mice
[67]. Of note, 8-weeks-long Se-deficiency lead to a significant reduction in the number of spermatogenic cells i.e., pachytene spermatocytes, late and mature spermatids. Intriguingly, the effect of Se-deficiency was less intense on germ cell kinetics at the early stages, suggesting a potential implication of Se-deficiency in affecting the overall sperm quality parameters observed in other similar studies
[67]. It has been suggested that loss of AP-1 (cJun/cFos) expression during the meiotic stages might have an involvement in preventing the progression of these gem cells to differentiate into spermatids
[68]. It has also been demonstrated that Se-deficiency, and to a lesser degree, Se-excess perturb these regulatory processes. As for the impact of high Se levels on modulation of AP-1, it has been suggested that Se at higher levels react with GSH and thiols present in proteins, contributing to the constitution of -S-S- and GS-Se-SG. Since the regulation of Fos and Jun is mediated by the redox status of a critical cysteine residue, thus the oxidation by excessive levels of Se may preclude the binding of cJun homodimers at the promoter sites and may lead towards the decreased
cjun mRNA
[68]. In addition, mitogen-activated protein kinase (MAPK) has been implicated in modulating
cfos gene expression, and high Se levels have been linked with inhibition of MAPK/c-Jun amino-terminal kinase (JNK) pathways. Therefore, these two mechanisms are suggested to have an involvement in decreasing the expression of
cjun and
cfos in condition of excess Se
[61]. Conversely, the reduced expression of these transcription factors in limiting Se conditions may be attributed to the increased ROS levels and subsequent oxidative damage at the promoter sites in these transcription factors, since the binding to AP-1 control sequence is reduced in Se-deficient conditions
[68].
Similarly, NFκB, a widely known redox-regulated transcription factor reportedly performs an important function in spermatogenesis. It has been shown that an increased relative expression of both
p65 and
p50 genes (components of NFκB) was observed in male mice consuming Se-deficient diet (0.02 ppm inorganic Se)
[66]. Besides, the levels of inducible nitric oxide and expression of inhibitory protein IκB were increased and decreased, respectively in Se-deficient mice
[66]. Previously, it has also been suggested that during conditions of oxidative stress in testis, NFκB proteins paly a pro-apoptotic role in spermatogenic cells, which elicits the hypothesis that an increased expression of NFκB might lead to an exuberant germ cell death and reduced fertility. Therefore; these findings clearly demonstrate that the decreased Se supply has potential negative implication on reproductive efficiency and spermatogenesis in mice via modulating the expression and activation of NFκB in testes
[66]. Intriguingly, it has been demonstrated that Se-mediated oxidative stress also has a potential implication in the modulation of expression of HSP70, HSP70-2, and MSJ-1 (a chaperone partner of spermatogenic cell-specific HSP70-2), contributing to an impaired spermatogenesis and reduced fertility in mice
[69]. Previously it has also been demonstrated that the alterations in Se-levels (deficiency or excess) could lead to significantly increased apoptosis (p53-meidated) in spermatogenic cells in mice
[65]. Therefore, these findings and others described before lend reasonable insights and add new dimensions to the understanding of underlying Se-mediated molecular mechanisms implicated in impairing the process of spermatogenesis and male fertility. However, the exact mechanisms behind such detrimental effects of alterations in Se-levels are still incompletely understood and require further elucidation.
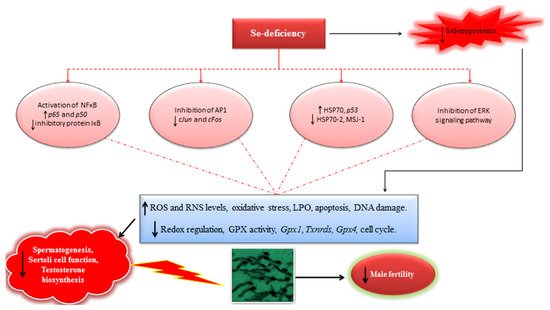
In light of available evidences, it could be inferred that Se-deficiency has a strong implication in modulation of redox signaling and intracellular oxidative stress, triggering the expression of several redox-sensitive transcription and proliferation factors, and ultimately affecting the process of spermatogenesis and male fertility (
Figure 1). Therefore, well-powered studies focusing on the elucidation of Se-mediated modulation of transcription and proliferation factors should be continued in future. Besides, it is also envisaged that like GPX4 and SELENOP, other selenoproteins may also have certain complementary roles in steroidogenesis and spermatogenesis in males. For instance, in situ hybridization experiments have demonstrated high levels of
Selenov mRNA in seminiferous tubules in mice, but its exact function in spermatogenesis still remains largely unexplored. Recently it has been shown that selenoprotein U (absent in mouse and human) has a potential implication in modulation of phosphatidylinositide 3-kinases–AKT–mechanistic (or mammalian) target of rapamycin (PI3K–AKT–mTOR) signaling pathway in chicken Sertoli cells (SC) and could perform a novel regulatory role in the SC-mediated spermatogenesis
[70]. Therefore, similar studies focusing on the possible physiological and regulatory functions of other selenoproteins such as, selenoprotein K (SELNOK), selenoprotein F (SELENOF), selenoprotein S (SELENOS), SELENOW, and others in mammalian counterparts may also provide enticing results and improve our understanding regarding Se and selenoprotein-mediated modulation of reproductive function in mammalian males. Moreover, it has been reported that seasonal variations could also influence the production of sperm. Therefore, it is highly recommended that replication of these findings in adequately powered studies (involving higher domestic animals) are needed to gain confidence with regards to the interpretation and comparison of evidences currently at hand. Meanwhile, such studies should also focus on an adequate (multiple) number of spermatogenesis cycles for ensuring the optimal effects of Se supplementation on steroidogenesis and spermatogenesis.
Figure 1. Schematic illustrating the implication of Se-deficiency in steroidogenesis, spermatogenesis, and male fertility (For details see the text in
Section 3.1).
3.2. Implication of Se on Male Fertility-Related Parameters
It has been reported that sperm maturation process has a strong association with the sperm and ejaculate quality, and overall reproductive efficiency in males. Therefore, any abnormality in such processes might result in ejaculates of inadequate and poorer quality and declined fertility in males
[14]. Similarly, increased Se dietary intake has been implicated in enhancing the antioxidant GPX activity, thereby improving the fertility in males
[15]. Meanwhile, as discussed before, an excessive supplementation could also result in no therapeutic advantage or even deleterious effects on overall reproductive outcome in males.
It is also worthwhile to mention that, even though the overproduction of ROS results in oxidative stress-induced DNA damage and/or apoptosis, membrane peroxidation and decreased sperm motility, an optimal level is necessary to carry some vital sperm functions viz. capacitation and acrosome reactions
[7]. Therefore, a delicate balance in the redox regulation is necessary for the optimal functioning of cells. It has been shown that the alterations in Se-levels may perturb the redox status and could lead to oxidative stress, adversely affecting male fertility by altering the expression of biologically important markers and activity of antioxidant enzymes. To this effect, Kaushal and Bansal
[69], reported that the alterations in Se-levels perturb the expression profiles of HSP70 proteins (heat shock proteins) and induce the oxidative stress. When mice were fed Se-deficient (0.02 ppm inorganic Se) and Se-excess (1.0 ppm inorganic Se) diets, a significant increase was observed in the levels of oxidative stress-related markers such as LPO, malondialdehyde (MDA) and free radicals (reactive oxygen species). Besides, a decreased level of GPX was noticed in Se-deficient mice. Conversely, Se-excess group showed relatively higher levels of GPX. Interestingly, the overall fertility-related markers were significantly diminished in both groups
[69]. In another similar study, increased LPO, elevated oxidative stress, and reduced levels of GPX were observed in male mice consuming Se-deficient diet
[66]. Recently, it has been demonstrated that two months long dietary supplementation of organic Se (0.3 mg per kg body weight) significantly improved the gross morphological and histomorphologcal indices in testes of young male goats. The enzymatic activity of GPX and superoxide dismutase (SOD) in serum and testicular tissue were also significantly ameliorated in young male goats treated with Se compared to the control group
[71].
In addition, Stefanov and colleagues
[72] studied the effects of organic (1.83g L-SeMet [Sel-Plex] per animal per day) and inorganic (4.0 mg sodium selenite per animal per day) Se supplementation on semen parameters in Bulgarian Merino rams. After 45 days of Se dietary supplementation, improvements were observed in semen volume per ejaculate, sperm motility and overall sperm survival rate in treated rams
[72]. Besides, in a very recent study on a mouse model, Asri-Rezaei and colleagues
[73] observed that an intraperitoneal injection of sodium selenite (0.50 mg per kg body weight) and Se nanoparticles (0.50 mg per kg body weight) for seven consecutive days adequately improved the tissue Se concentration in testis (as observed on day 28 post-injection). Similarly, the enzymic activities of antioxidant biomarkers were also significantly improved following Se treatment. In addition, the sperm quality parameters such as total count and motility were also improved compared to the control group
[73]. In general, these results corroborate that Se supplementation can produce beneficial effects and counter the oxidative stress.
Besides, recently in an interesting cost–benefit analysis
[74], it has been shown that boars fed organic Se-based (0.5 mg per kg) diet produced around 23% higher doses of semen per week compared to those maintained on inorganic Se diets (0.5 mg per kg). A significant increase in revenue (26%) was also observed in this study
[74]. In any case, additional animal studies reporting the effects of Se supplementation on the male reproductive efficiency in different animal models are presented in
Table 2. Meanwhile,
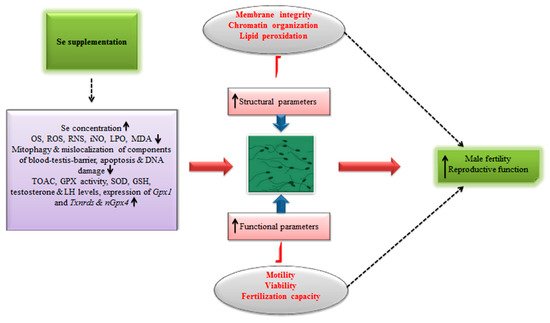
Figure 2 illustrates the implication of Se supplementation in ameliorating the male fertility and reproduction.
Figure 2. Schematic illustrating the implication of Se supplementation in ameliorating fertility and reproductive efficiency in males.
Table 2. Animal studies reporting the effects of selenium supplementation on male reproductive efficiency.
| Model |
Treatment |
Key Observations Reported |
Ref. |
| Sprague-Dawley rats |
Se nanoparticles at supranutritional levels (0.2, 0.4, or 0.8 mg Se per kg body weight) |
Sperm parameters such as, sperm concentration, motility, and morphological features were all improved at supranutritional levels. However, these parameters were significantly affected when rats were supplemented with higher levels (nonlethal level) of Se nanoparticles i.e., at 2.0, 4.0, or 8.0 mg Se per kg body weight. |
[75] |
| Sprague-Dawley rats |
Treated with inorganic Se [0.01(deficient); 0.25 (adequate); 3 (excess); or 5 (excess) mg per kg] for four weeks |
The U-shaped response of dietary Se was observed on DNA damage and sperm quality. Se deficiency showed a lower expression of sensitive antioxidant selenoproteins (Gpx1 and Txnrds). However, excessive doses of Se impaired sperm quality and this was linked with reduced mRNA expression of nGpx4. |
[76] |
| Mouse |
Se-supplement (inorganic Se (0.3 μg/g Se) or organic Se-enriched probiotics (containing 0.3 μg/g Se) given for 75 days |
Organic Se co-supplemented with probiotics significantly improved male fertility in mice. The ameliorated fertility index included the parameters such as, reduced testicular tissue injury, increased levels of serum testosterone, and improved sperm indices in Se-supplemented group. As such, these improved fertility-related parameters were ascribed to be the result of the antioxidant function of Se. |
[77] |
| Mouse |
0.2 ppm sodium selenite;
1.0 ppm sodium selenite |
Mice in both groups showed an increased occurrence of mitochondria- and plasma membrane-related defects, and DNA damage in sperm. However, these damages were more pronounced in mice exposed to Se-deficient feed. |
[78] |
| Mouse |
Se-deficient diet (0.02 ppm)
Se-sufficient (0.2 ppm); organic Se |
Sperm from Se-deficient mice demonstrated vitiated chromatin condensation, declined in vitro fertilization ability and increased lipid peroxidation (LPO) in both testes and sperm compared to the Se-sufficient mice. |
[79] |
| Mouse |
Se-deficient (0.02 ppm)
Se-excess (0.2 ppm); yeast-based Se.
Mice were fed for 4 months |
Se concentration and GPX activity (in testis) were significantly reduced. The fertility percentage and size of litter were both reduced in Se-deficient group. |
[61] |
| Aged mice |
Inorganic Se 0.2 mg/kg body weight |
Improved sperm parameters and increased expression of CatSper genes were observed in Se-treated group. |
[80] |
| Rabbit |
Treated with Se nanoparticles (400 μg/kg) for 60 days |
Improved serum testosterone levels were recorded in Se-treated group compared to the control. Besides, improved ejaculate volume and sperm quality parameters such as, sperm morphology, viability were observed. |
[81] |
| Ram |
0.5 ppm organic Se; 0.2 ppm organic Se |
A significantly higher concentration of Se and improved ejaculate and sperm quality were observed in seminal plasma of rams exposed to a feed containing 0.5 ppm organic Se compared to those who received 0.2 ppm organic Se. |
[82] |
| Boar |
Organic Se
(0.2 mg per kg);
Inorganic Se
(0.2 mg per kg) |
Ejaculate quality and sperm parameters were significantly improved in boars following dietary supplementation of organic Se (0.2 mg per kg) compared to those treated with sodium selenite at the same dose. |
[83] |
| Aardi buck |
Sodium selenite 0.1 mg/kg,
Sodium selenite 0.05 mg/kg |
Improved sperm count and motility was observed in both Se-treated groups. However, relatively better outcomes were observed in 0.1 mg/kg group. |
[84] |
| Boar |
0.5 ppm organic Se |
Following 11 weeks of feeding trail, organic Se supplementation increased glutathione peroxidase 4 (GPX4) activity (raw semen) and number of seminal doses in boars. |
[85] |
| Boar |
0.3 ppm organic Se;
0.3 ppm inorganic Se |
Following 12 weeks of Se supplementation, Se content and GPX activity were increased in semen of boars treated with organic and inorganic Se. Besides, semen quality parameters namely semen concentration and progressive motility of sperm were improved compared to the control group without Se. Improved resistance of liquid stored semen to hypo-osmotic shock and thermal tests, and improved fertility rates were observed in semen of boars treated with Se. All mentioned indices were slightly higher in the organic Se group compared to the inorganic group. |
[86] |
| Buffalo bulls |
10 mg organic Se/animal twice a week;
and
10 mg inorganic Se/animal twice a week |
Three months long Se supplementation significantly improved the sperm quality parameters (ejaculate volume, sperm motility, concentration, and morphology) in buffalo bulls. Besides, testosterone concentrations were also increased in Se-treated groups. |
[87] |
| Saanen bucks |
Inorganic Se 0.34 mg/kg body weight supplemented at ten-day intervals for three months |
Se supplementation improved the testicular biometry and sperm parameters. GPX activity, plasma testosterone and LH levels significantly increased in Se-treated group from days 40 to 80 compared to the control group. These indices reached peak reached peak at day 80 of the trial. |
[88] |
| Bovine bull |
In vitro fertilization (IVF) medium supplemented with Se (100 ng/mL) |
A significant increase in sperm mitochondrial activity was observed after 1 h of incubation in Se-supplemented IVF medium. Moreover, Se supplementation after 2 h of incubation showed an increase in HOST-positive (hypo-osmotic swelling test) sperm and sperm acrosome integrity. Increased number of sperm bound to zona pellucida (ZP) was observed in Se-treated group compared to the control. |
[89] |
It is reiterated that sperm quality parameters such as, sperm concentration, vitality, progressive movement, and overall sperm morphology are regarded as the essential clinical markers for the assessment of reproduction efficiency. Therefore, perturbations in these markers may lead towards sub- and infertility in males. Of note, oxidative stress has been implicated as an important factor contributing towards male infertility
[90]. Intriguingly, it is believed that sperm are more sensitive to oxidative damages; this is largely because of the biochemical composition of sperm i.e., it contains higher ratio of polyunsaturated fatty acids and low concentrations of cytoplasmic antioxidant enzymes compared to the somatic cells
[91]. Therefore, in conditions of increased ROS-induced oxidative stress, the plasma membrane integrity is affected due to the triggering of LPO cascades. Similarly increased oxidative stress is also implicated in inducing DNA damage in sperm by causing perturbations in chromatin condensation, and the process of chromatin condensation is an essential step for both sperm maturation and fertilization capacity
[79]. In this connection, it has been demonstrated that Se deficiency could lead to the impaired chromatin condensation and reorganization processes via elicitation of oxidative stress, and lead to the impaired sperm quality and reduced fertilization capacity in males
[79]. Nevertheless, in addition to aforementioned observations of previous studies, further studies focusing on the elucidation of potential markers of DNA integrity will be of high value and should lend encouraging insights with regards to the role of Se in process of spermatogenesis and overall male fertility. Besides, in-depth evaluation of sperm chromatin condensation and reorganization is also biologically more relevant because it is completely reorganized during the later phases of spermatogenesis when histones are substituted by protamines
[79]. Further adequately powered studies on Se-deficient models would also improve our understanding in this domain. As demonstrated in previous studies, Se deficiency could impair the sperm quality parameters and antioxidant status in the male reproductive organs, therefore it is reasonable to infer that Se-deficiency potentially perturbs the biosynthesis of selenoproteins having essential roles in redox regulation, leading towards oxidative insult and resultant reduced male fertility parameters. Therefore, in addition to already known mechanisms, more focus should be centered on elucidation of other putative mechanisms implicated in impairing the selenoprotein-mediated redox homeostasis in sperm/ejaculates.
The results of relevant studies with regards to the form of Se used are comparable and encouraging i.e., it is fairly evident that both organic and inorganic forms have the potential to ameliorate male fertility parameters in different animal models (also see Table 2). In order to get a more precise picture, it is also reasonable to conduct more adequately powered studies focusing on the comparative ameliorative effects of different forms of Se. It should also be considered that the effects of Se (especially the organic form) might be impacted by the duration of supplementation; therefore, for obtaining the desirable outcomes with regards to the improved fertility in different animal species, future studies should also include this aspect within their scope. Se-nanoparticles are also reported to possess more bioavailability and less toxicity potential compared to other conventional forms of Se. However, the level of evidence regarding the effects Se-nanoparticles in ameliorating male fertility is still insufficient, and more multifaceted studies are awaited to lend the concrete evidence.
Selenium in Seminal Plasma and its Implication in Male Fertility
Seminal plasma is considered to play many essential roles in motility, viability and sustentation of fertilizing ability of mammalian sperm
[92]. Villaverde et al.
[50] studied the correlation between the serum concentrations of Se and sperm quality traits, testosterone levels, and testes morphology in domestic cats. Se concentrations showed no influence on testosterone levels and sperm production. However, Se concentrations in blood and seminal plasma showed a correlation with sperm quality parameters. It was demonstrated that Se concentrations in seminal plasma were negatively correlated with total testicular weight and sperm morphological traits such as total head defects. Besides, Se concentrations in serum were also negatively correlated with some sperm motility-related parameters including average path velocity, straight line velocity, and curvilinear velocity
[50]. These observations, to some extent, highlight that Se (in seminal plasma) may have a potential implication in male fertility and could be used as an important marker in ameliorating the overall male reproductive biology of domestic cats
[50]. However, it should be noted that a small number of cats (
n = 6) was used in this investigation; therefore, inclusion of Se concentration (as a biomarker) in studies focusing on evaluation of potential association between this trace element and sperm quality and fertility in cats should be very cautiously assessed. In addition, for getting reasonable evidences, more data should be gathered from well-powered functional/mechanistic studies using the cat models. Similarly, Bertelsmann and colleagues
[93] determined the levels of Se in semen, seminal plasma and sperm, and their association with overall sperm quality and fertility in male horse. It was reported that Se level/concentration in sperm was correlated with sperm quality parameters such as, membrane integrity, progressive motility of sperm, positive acrosomal status and pregnancy rate per estrus cycle. Moreover, Se was also linked with ameliorated sperm quality and fertility in horse. These findings therefore suggest that evaluation of an optimal Se status for equine male reproduction necessitates the analysis of Se in sperm
[93].
It is worthwhile to mention that Se in seminal plasma originates from secretion of glandular epithelium of accessory sex glands i.e., prostate gland, seminal vesicles, and epididymis, therefore, alterations in seminal plasma Se-levels indicate that supply of Se to these sex glands changes with dietary Se. It was also demonstrated that effects of Se on sperm motility are likely arbitrated by secretions from these sex glands, either during the process of sperm maturation or at the time of ejaculation
[94]. Therefore, it is also reasonable to conduct more research on elucidation of potential functions of Se in seminal plasma and accessory sex glands. This will also improve our current understanding regarding underlying mechanisms and physiological basis of relationship between seminal plasma-Se concentrations and male fertility.
3.3. Combinatorial Effects of Se (as a Part of Micronutrient Supplement) on Male Fertility Outcomes (Animal Studies)
Recently, very few studies have demonstrated that Se in combination with other trace minerals and micronutrients can ameliorate the fertility-related outcomes in different animal species. To this effect, in one recent clinical study, Domosławska and colleagues
[95] demonstrated that Se-yeast (6 μg per kg) combined with vitamin E (5 mg per kg) supplement (treated for 60 days) significantly ameliorated the semen quality parameters and antioxidant status in clinically healthy dogs with reduced fertility. In Se-supplemented group, significant improvements were observed in parameters such as, blood Se concentration, mean sperm concentration, mean total sperm count, sperm motility, and percentage of live and normal sperm. Furthermore, sperm GPX activity and total antioxidant capacity (TAOC) were also increased significantly
[95]. Similar findings were also observed by the same group of researchers in 2015
[96], where the dietary supplementation of Se-yeast (6 μg per kg) in combination with vitamin E (5 mg per kg) for 60 days resulted in the improved sperm quality parameters such as total sperm count, concentration, morphology, and motility scores in clinically healthy dogs with reduced fertility
[96]. Similarly, Butt et al.
[97] demonstrated that Se (supplemented as 3g Selemax
®) in combination with vitamin E significantly improved the ejaculate and sperm quality parameters, and increased the concentration of testosterone in Holstein Friesian bulls maintained in a hot and humid environment
[97]. Additionally, Ghorbani et al.
[98] evaluated the effects of dietary Se (0.3 mg/kg), Zn (40 mg/kg) and their combination on reproductive performance in Sanjabi rams during the breeding season. They reported a significant improvement in sperm concentration, total sperm count, sperm motility following a 120 days long dietary supplementation. However, the concentration of testosterone remained unaltered
[98]. In another randomized, double-blinded study, Kirchhoff and colleagues
[99] failed to elucidate a clear trend in the extent to which a three months long supplementation of Se/vitamin E (tablets containing 0.1 mg organic Se; capsules containing 100 mg vitamin E) influences the semen qualitative traits in normospermic Cairn Terriers. Nevertheless, an effect of supplementation (head sperm defects) and a substantial interaction between time and treatment was observed for some semen parameters such as, percentage of viable sperm, sperm head irregularities, defective acrosome, and proximal cytoplasmic droplets. Nevertheless, it is worthwhile to mention that the number of dogs (
n = 9; three dogs per treatment group) used in this randomized study was too low and the subjects included were normospermic i.e., without any apparent semen quality defects. In any case, results of three recent studies (2016–2019) reporting the effects of combination treatment of Se and other micronutrients are summarized in
Table 3.
Table 3. Recent animal studies reporting the implication of selenium supplementation (in combination with other micronutrients) on male fertility outcomes.
| Animal Model and Number |
Treatment Regime and Duration |
Key Findings |
Ref. |
Male CD-1 mice
(n = 12 per experimental group) |
Fertilix® (CellOxess, Princeton, NJ, USA) was supplemented for two months.
(Se 55 μg, zinc 7.5–11 mg,
Full spectrum natural vitamin E 104–290 mg,
Lycopene 7.5–15 mg
Carnitine blend 200–800 mg
Folic acid 400–500 mg
Vitamin C 30–90 mg). |
Eight weeks long pretreatment with the antioxidant formulation completely protected oxidative stress-induced DNA damage in Gpx5 KO mice sperm. In mouse models of scrotal heat stress, only 35% (19/54) of female mice became pregnant resulting in 169 fetuses with 18% fetal resorption (30/169). Conversely, in antioxidant pretreated group 74% (42/57) of female mice became pregnant, resulting in 427 fetuses with 9% fetal resorption (38/427). |
[91] |
| Four infertile male dogs with low blood Se levels (86.0–165.0 μg/L) |
Organic Se 0.6 mg/kg and vitamin E (5 mg/kg) orally supplemented for 60 days. |
Treated dogs showed improved sperm parameters. Increase in blood Se concentration (401 μg/L) was observed at the end of trial. When these dogs were used for matting purpose, bitches successfully conceived and gave birth to 4–6 pups. |
[100] |
| Sixteen healthy normospermic dogs (two patients were excluded after adaptation period) |
A supplement comprising of Se 0.27 mg/kg vitamin E 250 mg/kg, vitamin B9 1.5 mg/kg, zinc 180 mg/kg, and n-3 PUFA 0.5%, given for 90 days. |
In treated group, sperm quality parameters i.e., total sperm count, concentration, sperm vitality and membrane integrity were significantly improved compared to the control group. |
[101] |
In general, the results of these studies are encouraging and suggest that Se in combination with other essential micronutrients could improve the reproductive efficiency in males. However, the level of concrete evidence is still insufficient and somehow inconsistent. Therefore, well-powered randomized studies will be of high value for building the solid scientific evidence in this regard. It should also be noted that these ameliorative effects of integrated mixture of essential micronutrients might be a result of augmentative and camouflaged effects of each essential micronutrient
[101]. Currently, the underlying mechanisms of such combinatorial effects are largely unclear and should be considered in future investigations
[35]. Nevertheless, such studies will also lend reasonable basis for determining the proper and adequate remedy protocols aimed at ameliorating male factor sub- and infertility in mammalian species, particularly in domestic and companion animals.