2. Chronic Kidney Disease and Endothelial Dysfunction
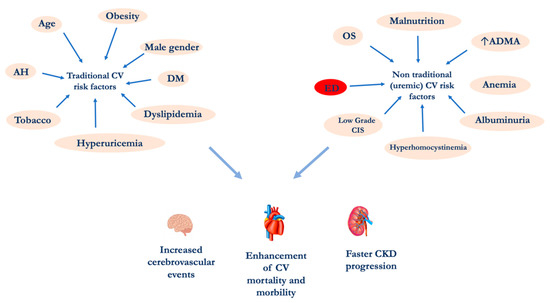
Chronic nephropathy is a systemic disease that currently affects about 10% of the general population
[27]. The CKD increased prevalence is certainly due to a global ageing of the population and especially to the increase in the incidence of metabolic diseases such as diabetes mellitus (DM), metabolic syndrome (MetS), obesity, etc.
[28].
The association between CKD and CVD was first highlighted in end-stage renal diseases (ESRD) patients in dialytic treatment
[2]. More recently, it has been shown that even the presence of slight changes in renal function, such as albuminuria, leads to a significant enhancement in CV events, which are more frequent compared to the progression versus ESRD.
CKD causes ED through several mechanisms. Physiologically, the vascular endothelium can be considered a “real organ”, able to secrete, in response to a great variety of signals, numerous chemical mediators
[29][30]. Among the main physiological functions of the endothelium, the nitric oxide (NO) release represents a milestone. This molecule displays several functions in the human body such as the regulation of neuronal communication, inflammatory and immune responses, and the regulation of vascular tone
[31][32].
ED represents an abnormality that develops at the level of the tunic that covers the internal surface of arterial and venous vessels and more precisely an alteration of normal endothelium, which results in the loss of some structural and/or functional characteristics
[33][34].
In particular, at renal level it is possible to distinguish different types of endothelial cells, such as those of the small vessels that regulate the blood flow in the kidney and are also involved in the coagulation process and in the regulation of vascular permeability and in the inflammatory state. The glomerular endothelial cells, in addition to the already mentioned functions, are involved in glomerular filtration and in providing support to the podocytes. Finally, the endothelial cells of the microvasculature in the kidney tubules participate in the tubular reabsorption
[35][36]. As for the kidney dysfunction, it has been highlighted a transition of kidney endothelial cells into mesenchymal phenotype, phenomenon called endothelial-to-mesenchymal transition, promoting renal fibrosis. Therefore, in CKD patients the accumulation of uremic toxins worsens the residual renal function and induces the systemic ED, contributing to cause CV comorbidities
[37]. In fact, the increased incidence of CV events in CKD patients is related to the uremic toxins but also to the chronic inflammation state, typical of this pathological condition
[38]. The evidence of a close relationship between kidney and hearth lays the foundations for a wider and more detailed stratification of the CV risk and suggests the opportunity for a correct and prompt management of CV comorbidities, starting from the early stages of CKD
[39].
During CKD, the ED development appears to be related to a decrease in the inducible nitric oxide synthase (iNOS) activity and an increase in ADMA levels
[31]. The latter gradually increases with the decline of the renal function. In fact, ADMA concentration is related to CKD progression, and it is an important CV risk factor both in the general population and in nephropathic patients
[40]. ADMA is synthetized endogenously by the degradation of methylated proteins
[41]. The asymmetrical residues of methylated arginine such as ADMA itself and NG-monomethyl-L-arginine (L-NMMA), inhibit the NO synthesis, as they are competitive inhibitors of NO synthase (NOS). ADMA and L-NMMA are excreted in the urine by renal pathways, and in CKD patients it can be observed an enhancement ADMA levels
[42]. The ADMA accumulation in nephropathic patients could be related to renal parenchyma damage, resulting in a reduced expression and activity of dimethylarginine dimethylaminohydrolase (DDAH)
[43]. This enzyme is involved in ADMA metabolism, in fact DDAH induces the ADMA hydrolytic degradation into L-citrulline and dimethylamine
[44]. In some pathological conditions, such as DM, hyperhomocysteinemia, OS and dyslipidemia, DDAH reduced levels have been observed
[45]. A decreased activity of DDAH induces an increase in ADMA concentration and subsequently a decreased NO production in the endothelium
[46]. For this reason, ADMA has been described as a compound able to induce ED. In fact, intra-arterial local infusion of ADMA seems significantly reduce the blood flow in forearm
[47], while the intravenous one causes an enhancement of blood pressure values of 6% and of systemic vascular resistance of 24%
[48]. Another cause of ADMA accumulation in CKD patients, it is represented by an increased rate of protein turnover that, in turn, causes enhanced concentrations of methylated protein
[49]. A study by Damaso et al.
[50] conducted on a cohort of 225 HD patients, highlighted also an interaction between inflammation biomarkers and ADMA. In particular, the mortality was higher in the group that had both elevated ADMA and C-reactive protein (CRP) levels compared to groups that had only one of the biomarkers altered. The authors concluded that inflammation amplifies the risk of death and CV events in HD patients with ADMA high levels. Therefore, further studies are needed to establish normal serum and urinary ranges of ADMA for different ages. The link between ED, increased ADMA levels and OS is represented by the impaired NO production. In fact, NO, as above mentioned, performs several biological functions
[51]. The reaction catalyzed by NOS enzyme, starting from L-arginine, produces NO and L-citrulline
[52][53][54]. In blood vessels, endothelial NOS (eNOS) represent the most abundant isozyme. Under pathological conditions, eNOS can produce reactive oxygen species (ROS)
[55]. In turn, the OS can induce an alteration in NO production through two mechanisms involving eNOS enzyme: the first one is represented by the eNOS inhibition and the second one is due to uncoupling eNOS. Consequently, the decreased NO production contributes to cause ED
[56]. Among the factors related to OS during CKD, it should be considered the accumulation of uremic toxins such as indoxyl sulfate (IS), homocysteine (Hcy), and advanced glycation end products (AGEs)
[57][58].
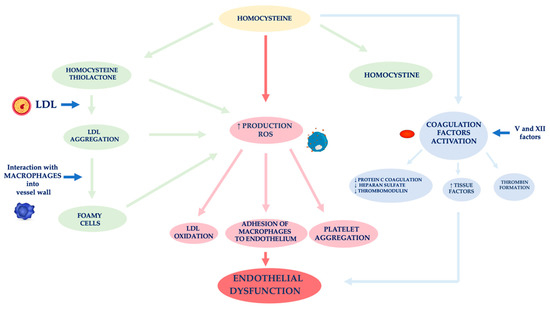
In nephropathic patients, another factor related to ED is hyperhomocysteinemia (
Figure 2)
[59]. Hcy is an amino acid sulfide, placed at the crossroad of a complex metabolic pathway. Normal Hcy levels are between 5 and 15 μmol/L
[60], while hyperhomocysteinemia is defined when Hcy concentration is above 15 μmol/L
[61].
Figure 2. Mechanisms of vascular wall damage induced by homocysteine. Abbreviations: LDL, low-density lipoprotein; ROS, reactive oxygen species.
Factors affecting an increase of Hcy plasma levels can be genetic and/or acquired. As for the former, several mutations have been identified in the various genes involved in Hcy metabolism. The most analyzed mutation is certainly that relating to the methylenetetrahydrofolate reductase (MTHFR) gene. The acquired factors include gender, age, kidney function and lifestyle
[62]. In fact, Hcy levels in CKD patients are on average increased because of its reduced kidney metabolism, namely between 20 and 40 μmol/L, especially if it is also present a folate and B12 vitamin deficiency, malnutrition, accumulation of uremic toxins
[63][64][65][66]. Several authors demonstrated that Hcy plasma concentration is directly related to plasma creatinine values as it has been shown that in the renal parenchyma there are enzymes involved in Hcy remethylation and transsulfuration
[67][68][69]. Jansen et al. hypothesized that hyperhomocyteinemia in CKD patients is mainly related to the slowing of its catabolic pathway of transsulfuration
[70]. In fact, the enzymes cystathionine-β-synthase and cystathionine-γ-lyase are located in the proximal tubules of the nephron. However, the Hcy excretion in the urine is limited to 0.1% of the Hcy total, as the latter is largely reabsorbed at the tubular level. Unlike cystine, which has an antioxidant action, Hcy has a pro-oxidant action, as it promotes the formation of free radicals. The different behavior of these two amino acids is related to both an Hcy auto-oxidation and its enzymatic transformation into thiolactone, a substance with a strong oxidizing power
[71]. The formation of thiolactone induces structural changes in intracellular proteins up to the loss of the biological activity of native proteins. The thiolation process can also occur at level of extracellular components, such as apolipoprotein B (apoB) and low-density lipoprotein (LDL). Hcy forms, with the LDL, aggregates which are captured by macrophages in the vessel wall. These phagocytic cells are transformed into foamy cells that release other free radicals that induce the oxidation of LDL, the platelet aggregation and the adhesion of macrophages to the endothelium
[72][73][74]. Meanwhile, the Hcy autoxidation induces the production of further free radicals (hydroxyl) that trigger a process of lipid peroxidation at the level of the endothelial membranes
[75][76].
ED induced by Hcy is also caused by the activation of coagulation factors, such as V and XII, whose stimulation induces: (i) a reduced expression of the coagulation protein C, of the thrombomodulin and of the heparan sulfate by the endothelium, (ii) an increased expression of the tissue factors and the thrombin formation
[77]. In physiological conditions, the endothelium develops defensive mechanisms against the toxic action induced by hyperhomocysteinemia, releasing NO which forms S-nitrous-homocysteine, a compound that inhibits the production of hydrogen peroxide. S-nitrous-homocysteine has an important vasodilating action and inhibits platelet aggregation
[78]. However, the persistence of the damage produced by a chronic condition of hyperhomocysteinemia, progressively reduces the ability of the endothelium to produce NO. An in vitro study by Tyagi et al. showed that Hcy activates the protease-activated receptors 4 which induces the production of ROS through an increase in NADPH oxidase and a decrease in expression of thioredoxin. Hyperhomocysteinemia also reduces the bioavailability of NO through the increase in NO
2-tyrosine and through the accumulation of ADMA caused by the reduced expression of DDAH
[79].
Furthermore, Hcy appears to stimulate the release of interleukin (IL)-8 and macrophage chemotactic factor (MCF). These chemokines have specific chemotactic activity for monocytes and neutrophils. The infiltration of the arterial wall by monocytes is a key event for the induction of atherogenesis. Instead, the monocyte chemoattractant protein (MCP) stimulates the migration of monocytes into the intima of the vessel wall. The OS induced by Hcy, on the one hand, directly damages endothelial cells, and on the other hand, induces the expression of matrix metalloproteases (MMPs), enzymes responsible for remodeling the vessel wall. In physiological conditions, MMPs are in equilibrium with their inhibitors, while in pathological conditions, this equilibrium is unbalanced towards a decrease in MMPs contextual to the increase in their inhibitors
[80][81].
Another mechanism causing ED in CKD is an unbalance of the calcium-phosphorus metabolism. In particular, phosphate retention induces the development of CKD-mineral bone disorder (CKD-MBD) which, in turn, contributes to cause vascular calcifications. In fact, numerous epidemiological studies have shown that higher serum phosphorus levels, even in the absence of CKD, represent a risk factor for CVDs
[82][83]. During CKD, vascular calcifications of the intima and media are frequent and are correlated to vascular rigidity. Elevated phosphate levels trigger the transformation of smooth muscle cells of the arterial wall into an osteoblast-like phenotype
[84][85]. In addition, hyperphosphatemia affects ED, increasing apoptosis, inducing an increased production of ROS, impairing the NO production and decreasing the expression of annexin II
[86][87]. The latter is a glycoprotein involved in various cellular functions, including the motility of epithelial cells, the fibrinolysis, the formation of anion channels and the interaction with matrix cells
[88].