1000/1000
Hot
Most Recent

Plants continuously rely on light as an energy source and as the driver of many processes in their lifetimes. The ability to perceive different light radiations involves several photoreceptors, which in turn activate complex signalling cascades that ultimately lead to a rearrangement in plant metabolism as an adaptation strategy towards specific light conditions. This entry introduces the main classes of secondary metabolites and specifically focuses on the influence played by the different wavelengths on the content of these compounds in agricultural plants, because of their recognised roles as nutraceuticals.
Plants rely on an uncountable number of secondary metabolites during their lifespans in order to perform several fundamental functions, such as attracting pollinators, mechanical support, protection from solar UV radiation, deterrents against pests, pathogens, and herbivores, interaction with other plants, and response to environmental stimuli/stresses [1]. Thanks to a network of photoreceptors and the following complex signalling routes, the different light wavelengths may impact the content of these metabolites by up- or downregulating specific sets of biosynthetic and regulatory genes.
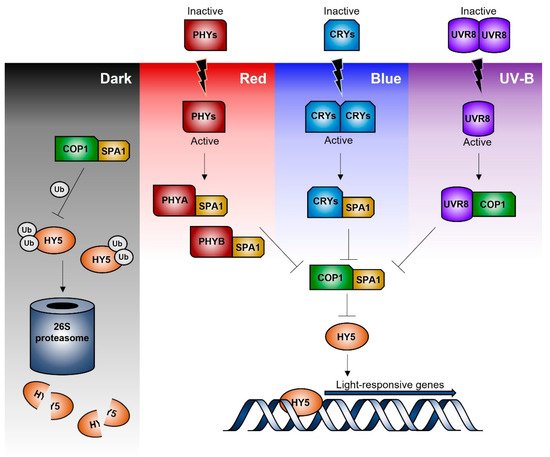
Independently from the light quality and kind of photoreceptor involved in light perception, the downstream event proceeds via a complex network of early signalling factors, central integrators, and final effectors. Please refer to some recent reviews [2][3][4][5] for a detailed summary of the current knowledge of the transcriptional network and mechanisms regulating the response to the different light spectral composition. Interestingly, CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1), which promotes the proteasome-mediates degradation of key factors involved in light signalling, is involved in the response to any light radiation, from UV to far-red wavelengths [6]. Similarly, the transcription factor ELONGATED HYOCOTYL 5 (HY5) has a central role as a final effector of all the light-dependent signalling routes, being able to bind to the promoters of about 4000 genes in Arabidopsis [6].
Figure 1 represents a simplified scheme of the signal transduction pathways leading to gene regulation in response to blue, red/far-red, and UV-B radiation. Briefly, under dark conditions, COP1/SPA (suppressor of Phytochrome A) ubiquitin ligase complex promotes the ubiquitination and degradation of HY5 via the 26S-proteasome pathway [4]. Upon light perception, the active blue- and red/far-red-photoreceptors (cryptochromes and phytochromes) interact with the COP1/SPA complex binding to SPA; thus, leading to COP1 disassembly and migration outside the nucleus. This prevents HY5 ubiquitination and subsequent degradation, so that HY5 may bind the promoter sequence of the light inducible target genes. Similarly, UVR8, after UVB-induced monomerization, can bind to COP1, leading to a functional disruption of the COP1/SPA complex and a consequent HY5 stabilization and functioning [7][8].
This review specifically focuses on the influence of the different light radiations, from red–far-red to UV-B, on the main classes of secondary metabolites, such as phenolic compounds, terpenoids, tocopherols, glucosinolates, and ascorbic acid in agricultural plant species, because of the recognised role that these compounds generally play as promoters of human wellness [9][10][11][12]. UV-C radiation was reported to modulate accumulation of health-promoting compounds in different plants and fruits of food interest, such as tomato fruit [13], bean seedlings [14] and peanut sprouts [15], this review exclusively discusses the effects of those wavelengths that reach the Earth’s surface, and to which plants have adapted fine-tuning perception mechanisms and consequent molecular and biochemical responses through evolution.
According to their chemical structures, terpenoids fulfil essential functions during plant life as, e.g., direct/indirect defensive compounds against biotic stressors, deterrent towards herbivores, photosynthetic pigments, signalling molecules mediating plant-plant, and plant–environment interaction [16][17][18][19].
Vitamin E has been widely studied due to its high antioxidant activity, especially preventing the oxidation of mono- and poly-unsaturated lipids. In addition, vitamin E compounds were shown to have hypolipidemic, antiatherogenic, antihypertensive, neuroprotective, anti-inflammatory, and many other beneficial effects for human health [20][21][22][23][24]. The main plant sources of tocopherols and tocotrienols are seeds (especially oilseeds) and nuts. In addition, they can be found in many plants and fruits, although their concentrations are limited due to their low lipid content [25].
Vitamin C, like the majority of the hydrosoluble vitamins, participates as a cofactor for many enzymes, e.g., members of the mono- and dioxygenases family [26], essentially contributing to the maintenance of the cell redox state, together with several other antioxidant molecules and enzymes. In plants, vitamin C is involved in many pathways and processes, e.g., the xanthophyll cycle, the flavonoids, and the glucosinolates pathways, and in the biosynthesis of plant hormones, such as ethylene, gibberellins, and abscisic acid [27][28][29][30][31]. Studies on the role of vitamin C role and its benefits in humans started when it was first noticed that vitamin C deficiency determined a potentially lethal disease called scurvy [32], negatively affecting the immune system, the collagenous architecture, and the regeneration process from wounds. Moreover, pharmacological effects of ascorbic acid against cancer and cardiovascular diseases were also observed [33][34]. The main dietary sources of vitamin C are fresh fruits and vegetables; therefore, their consumption has been widely encouraged by the main food and health organisations (e.g., the Food and Nutrition Board of the National Academy of Sciences, the European Food Safety Authority (EFSA), and the Food and Drug Administration (FDA)) throughout the years, and vitamin C deficiency symptoms have progressively reduced worldwide.
Similarly, when bilberries ( Vaccinium myrtillus L.), plants were exposed to monochromatic red light (7.8 μmol m −2 s −1 ) during the berry ripening period, a significant increment of total anthocyanins occurred, due to the positive effect of this radiation on petudinins and delphinidins, while peonidins decreased, and cyanidins and malvidins were unaffected [35]. This finding underlines an interesting aspect of the light–phenolic interaction, i.e., the diversity of response to the same stimulus shown by different subclasses of molecules belonging to the same metabolic class. A similar phytochemical specificity of response was also observed in wheat ( Triticum aestivum L.) sprouts grown under a 16-h light/8-h dark photoperiod under white, red, or blue light, for up to 12 days. Specifically, red light, at the end of the growing period, did not lead to a significant increase in the content of total phenylpropanoids in comparison to white light, but modified their composition, inducing an increase in quercetin and a decrease in 4-hydroxybenzoic acid [36].
A 7-day exposure of red clover ( Trifolium pratense L.) sprouts to red-light (630 nm, 150 μmol m −2 s −1 , LEDs as lighting sources) induced a significant decrease in zeaxanthin concentration, while β-carotene and lutein were unaffected by the treatment [37]. A negative impact of this radiation on β-carotene concentration (−42.5%) was instead observed in Romaine green baby leaf lettuce (cv. Thumper) treated with supplemental red light (638 nm, 150 μmol m −2 s −1 , LEDs lighting sources) for 3 days [38]. These results differed from the ones by Li and Kubota (2009) [39], who found that 12 days of supplemental far-red light, but not red-light , irradiation determined a decrease in xanthophylls and β-carotene concentration in “Red Cross” baby leaf lettuce. Moreover, red light (380 μmol m −2 s −1 , LED lighting sources) was ineffective in modifying the carotenoid content of another lettuce cultivar (“Red Fire”) when compared to white light [40].
Tocopherols are reported to be influenced by red light. Exposure to supplemental red light (638 nm, 150 μmol m −2 s −1 , LEDs lighting sources, 3 days) was effective in significantly increasing α- and γ-tocopherols of Romaine green baby leaf lettuce (cv. Thumper) [38]. The same authors [41] also detected significant accumulation of α-tocopherol in basil microgreens grown under increased or sole red radiation (638 nm). However, increased red radiation lowered the α-tocopherol content of parsley microgreens, which was instead incremented when cultivation occurred with sole red lightning. It is therefore evident that, as observed for phenolic compounds, tocopherols are also influenced by red radiation in a species-depending way.
The limited data available on light quality influence on glucosinolates show that the effect is highly dependent on the wavebands and the plant species. Under red irradiation (730 and 640 nm) sinigrin content of kale was higher as compared to plants grown under blue light [42]. Similarly, when three Chinese cabbage varieties were exposed for 24 h to fluorescent light supplemented with red LEDs (625 nm), the content of total glucosinolates increased in the variety characterised by a low content of these metabolites, while the variety with high glucosinolates positively reacted to supplemental blue radiation [43]. These authors also reported that different set of genes involved in glucosinolates biosynthesis were upregulated by red or blue radiations in Chinese cabbage. To confirm the genotype dependence of the light influence on glucosinolates biosynthesis, Qian et al. [44] did not observe any variation in the content of these compounds in Chinese kale sprouts exposed to red LED light.
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Lettuce (Lactuca sativa. L.) | Youmaicai | ↑ | [50] | |||||||
| Butterhead | ↑ | [51] | ||||||||
| Tea leaves (Camellia sinensis L.O. Kuntze) | Zhonghuang 3 | ↑ | ↑ | [52] | ||||||
| Tomato plants (Solanum lycopersicum L.) | Komeett’ | =/↑ | [53] | |||||||
| Basil (Ocimum basilicum L.) | Improved Genovese Compact | =/↓ | =/↓ | =/↓ | =/↓ | [54] | ||||
| Red Rubin | =/↓ | =/↓ | =/↓ | =/↓ | ||||||
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant. | ||||||||
| Green leafy lettuce (Lactuca sativa. L.) | Thumper | ↓ | = | ↓/↑ | ↑ | [133] | ||||
| ↑ | [167] | |||||||||
| Grizzly | ↑ | [168] | ||||||||
| Red leafy lettuce (Lactuca sativa. L.) | Red Cross | = | ↑ | [131] | ||||||
| Red clover (Trifolium pratense L.) | ↑ | [144] | ||||||||
| Chinese cabbage (Brassica campestris L.) | ↑ | [169] | ||||||||
| Mustard (Brassica juncea L.) | Red Lion | ↑ | ↑ | [170] | ||||||
| Beet (Beta vulgaris L.) | Bulls Blood | ↑ | ↑ | |||||||
| Parsley (Petroselinum crispum Mill.) | Plain Leaved or French | ↑ | ↑ | |||||||
| Buckwheat (Fagopyrum esculentum) | Möench | ↑ | ↑ | [128] | ||||||
| Wheat (Triticum aestivum L.) | ↑/↓ | [145] | ||||||||
| Soybean (Glycine max L.) | Dongnong 690 | ↑ | ↓ | [166] | ||||||
| Bilberry fruit (Vaccinium myrtillus L.) | ↑ | [148] | ||||||||
| Apple fruit (Malus domestica Borkh.) | Mishima Fuji | ↑ | [171] | |||||||
| Jonathan | ↑ | |||||||||
| Strawberry (Fragaria × Ananassa) | ↑ | [172,173] | ||||||||
| Fengguang | ↑ | [174] | ||||||||
| Cowpea (Vigna unguiculata L. Walp.) | ↓/↑/= | [147] | ||||||||
| Tartary buckwheat (Fagopyrum tataricum Gaertn.) |
↓ | [146] | ||||||||
| Pak choi (Brassica rapa ssp. chinensis) | ↓/= | [150] | ||||||||
| Tomato fruit (Solanum lycopersicum L.) | Micro-Tom | ↑ | [175] | |||||||
| Satsuma mandarin fruit (Citrus unshiu Marc.) | ↓/↑/= | [152] | ||||||||
| Tea leaves (Camellia sinensis) | Jinxuan | ↑ | [149] | |||||||
| Basil (Ocimum basilicum L.) | Genovese | ↑ | [176] | |||||||
| Satsuma mandarin fruit (Citrus unshiu Marc.) | ↑ | [177] | ||||||||
| Valencia orange fruit (Citrus sinensis Osbeck) | ↑ | |||||||||
| Lisbon lemon fruit (Citrus limon Burm.f.) | ↑ | |||||||||
| Canola (Brassica napus L.) | ↑/= | [178] | ||||||||
| Mustard (Brassica juncea L.) | ↓ | [179] | ||||||||
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Pak-choi (Brassica rapa ssp. chinensis var. communis) | Red leaf cv. | ↑/= | ↑ | ↑ | ↑ | ↑/= | ↑/↓ | ↑ | ↑ | [55][56] |
| Green leaf cv. | ↑/= | ↑/= | ↑ | ↑ | = | ↓ | ↑ | |||
| Turnip (Brassica rapa subsp. rapa) | Tsuda | ↑ | [57] | |||||||
| Broccoli (Brassica oleracea L., var. italica) | Waltham 29 | =/↓ | =/↓ | [58] | ||||||
| Monopoly | =/↓ | = | [59] | |||||||
| Broccoli (Brassica oleracea L., var. gemmifera DC) | ↑/↓ | ↑/= | [60] | |||||||
| Lettuce (Lactuca sativa. L.) | Yanzhi | = | ↑ | ↑ | ↑ | ↓ | ↑ | [61] | ||
| Red butter | ↑ | ↑ | ↑ | = | ↓ | ↑ | ||||
| Klee | ↑/= | ↑/= | ↑ | ↑ | [62] | |||||
| Red leaf cvs. | = | [63] | ||||||||
| Green leaf cvs. | = | |||||||||
| Hongyeom | ↑/= | ↑/= | ↑/= | [64] | ||||||
| Tomato plant (Solanum lycopersicum L.) | Oxheart | ↓ | = | = | = | [65] | ||||
| Cherry | = | ↓ | ↓ | ↓/= | ||||||
| Roma | = | = | ↓ | ↑/= | ||||||
| MicroTom | ↑ | [66] | ||||||||
| Tomato fruit (Solanum lycopersicum L.) | Budenovka | ↑ | ↑ | ↑/= | [67] | |||||
| Bull Heart | ↑ | ↑ | ↑/= | |||||||
| Gina | ↑ | ↑ | ↑/= | |||||||
| Micro-Tom | ↑ | [66] | ||||||||
| Sowthistle (Ixeris dentata Nakai) | ↑/= | ↑/= | ↑/= | [68] | ||||||
| Grape berry (Vitis vinifera L.) | Cabernet Sauvignon | ↑ | [69] | |||||||
| Blueberry (Vaccinium corymbosum L.) | Duke | ↓ | = | [70] | ||||||
| Peach fruit (Prunus persica L. Batsch) | Hujingmilu | ↑ | [71] | |||||||
| Yulu | = | |||||||||
| Basil (Ocimum basilicum L.) | Genovese | ↑/= | ↑ | ↓ | ↑/↓ | [56][72][73][74][75] | ||||
| Beet (Beta vulgaris L.) | Bulls Blood | ↑/↓ | ↑ | [56] | ||||||
| Rice (Oryza sativa L.) | Kanchana | ↑ | ↑ | [76] | ||||||
| Mattatriveni | ↓/= | |||||||||
| Harsha | ↑/= | |||||||||
| Broccoli (Brassica oleracea L. var. italica) | Waltham 29 | = | ↑ | [77] | ||||||
| Wheat (Triticum aestivum L.) | Sumai188 | ↑ | [78] | |||||||
| Mung bean (Vigna radiata) | ↑/↓ | ↑ | ↑ | [79] | ||||||
| Peppermint (Mentha piperita L.) | Rubescens | ↑ | ↑/↓ | [80] | ||||||
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Basil (Ocimum basilicum L.) | Genovese | ↑/= | ↑/= | [72][73][74][75] | ||||||
| Cinnamon | ↑/↓ | ↑/=/↓ | [81] | |||||||
| Rice (Oryza sativa L.) | Kanchana | ↑ | ↑ | [76] | ||||||
| Mattatriveni | ↓/= | |||||||||
| Harsha | ↑/= | |||||||||
| Broccoli (Brassica oleracea L. var. italica) | Waltham 29 | = | ↑/= | ↑ | [58][77][82] | |||||
| Broccoli (Brassica oleracea var. gemmifera DC) | = | [60] | ||||||||
| Wheat (Triticum aestivum L.) | Sumai188 | ↑ | [78] | |||||||
| Mung bean (Vigna radiata) | ↑/↓ | ↑ | ↑ | [79] | ||||||
| Peppermint (Mentha piperita L.) | Rubescens | ↑ | ↑/↓ | [80] | ||||||
| Lettuce (Lactuca sativa. L.) | Red leaf cvs. | ↑ | ↑ | ↑ | ↓ | [63][83] | ||||
| Green leaf cvs. | ↑ | ↑ | ↑ | |||||||
| Peach fruit (Prunus persica L.) | Suncrest | ↑/=/↓ | = | ↓/↑ | =/↓ | [84][85] | ||||
| Big Top | ↑/=/↓ | ↓/↑ | ↑ | |||||||
| Babygold 7 | ↓ | ↓/↑ | = | |||||||
| Fairtime | ↓/↑ | ↓/↑ | ↓/↑ | ↓ | [86][87] | |||||
| Yulu | ↑ | [71] | ||||||||
| Hujingmilu | ↑ | |||||||||
| Tomato fruit (Solanum lycopersicum L.) | Money Maker | ↑ | ↑ | [88][89] | ||||||
| Zhenfen 202 | ↓ | [90] | ||||||||
| Bell pepper fruit (Capsicum annum L.) | Angus | ↑ | [91] | |||||||
| Green lime fruit (Citrus latifolia Tan.) | ↑ | [92] | ||||||||
| Spinach (Spinacia oleracea L.) | Meridian | ↑ | [93] | |||||||
| Maize (Zea mays L.) | ↓ | [94] | ||||||||
| Cucumber (Cucumis sativus L.) | Long green | ↓ | [95] | |||||||
| Apple fruit (Malus domestica Borkh.) | Aroma | ↑ | [96] | |||||||