1000/1000
Hot
Most Recent

Euphorbia genus (Euphorbiaceae family), which is the third largest genus of angiosperm plants comprising ca. 2000 recognized species, is used all over the world in traditional medicine, especially in the traditional Chinese medicine. Members of this taxa are promptly recognizable by their specialized inflorescences and latex. In this review, an overview of Euphorbia-derived natural products such as essential oils, extracts, and pure compounds, active in a broad range of biological activities, and with potential usages in health maintenance, is described. The chemical composition of essential oils from Euphorbia species revealed the presence of more than 80 phytochemicals, mainly oxygenated sesquiterpenes and sesquiterpenes hydrocarbons, while Euphorbia extracts contain secondary metabolites such as sesquiterpenes, diterpenes, sterols, flavonoids, and other polyphenols.
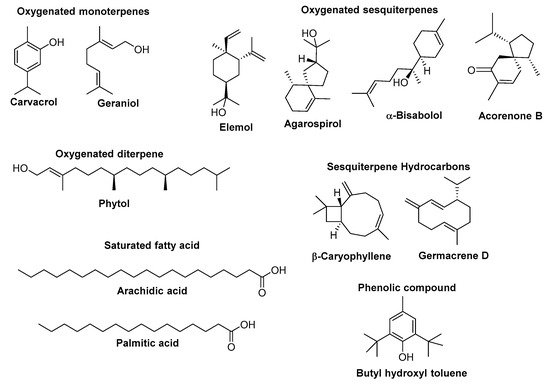
| Species | Origin | Raw Material | Extraction Method | Main Components a (%) | Most Relevant Biological Activities | Ref. |
|---|---|---|---|---|---|---|
| E. acanthothamnos Heldr. & Sart. ex Boiss. | Greece | Inflorescences | Steam distillation | Phytol (28.3), phytol acetate (9.3), β-caryophyllene (7.5) | not evaluated | [59] |
| E. apios L. | Greece | Inflorescences | Steam distillation | Germacrene D (30.0), heptacosane (12.7), β-caryophyllene (10.0), tricosane (6.5), pentacosane (6.0) | not evaluated | [59] |
| E. characias L. | Greece | Inflorescences | Steam distillation | Nonanal (22.8), phytol (13.5), pentacosane (8.5), heptacosane (7.4), palmitic acid (5.7), nonacosane (5.6) | not evaluated | [59] |
| E. cotinifolia L. (syn. E. caracasana (Klotzsch & Garcke) Boiss.) | Venezuela | Leaves | Hydro-distillation | β-Caryophyllene (39.3), germacrene-D (21.5%), α-copaene (9.3), α-humulene (5.2) | not evaluated | [60] |
| E. dendroides L. | Greece | Inflorescences | Steam distillation | Heptacosane (10.5), pentacosane (6.0), 4-terpineol (5.5), tricosane (5.0) | not evaluated | [59] |
| E. densa Schrenk | Syria | Aerial parts | Hydro-distillation | 1,8-Cineole (18.87), linalool (13.61), carvacrol (13.32), (E)-caryophyllene (10.29) | Radical scavenging activity (EC50 = 0.35 µg/mL) lower than BHA (EC50 = 0.135 µg/mL) | [61] |
| E. fischeriana Steud. | China | Roots | Steam distillation | Eudesmol (18.22), p-menth-8-en-2-ol (9.36), caryophyllene oxide (8.61), selinenol (6.83) | Radical scavenging activity (IC50 = 57.2 µg/mL) similar to ascorbic acid (IC50 = 63.1 µg/mL) but lower than BHT (IC50 = 26.1 µg/mL) | [62] |
| E. fragifera Jan | Italy | Inflorescences | Steam distillation | Carvacrol (61.55), carvon (9.22), β-caryophyllene (5.80)/geraniol (59.65), β-caryophyllene (9.05) | not evaluated | [63] |
| E. gaillardotii Boiss. & Blanche | Turkey | Aerial parts | Hydro-distillation | Arachidic acid (32), hexatriacontane (8.7), mint furanone (8.4), palmitic acid (8.0), tetratetracontane (6.2), octadecane (5.6), α-silenene (5.2) | Anti-lipid peroxidation activity (IC50 = 14.8 µg/mL) similar to α-tocopherol, but much lower radical scavenging activity than BHT. | [64] |
| E. golondrina L.C.Wheeler | Cameroon | Leaves | Steam distillation | Caryophyllene oxide (14.16), 2-pentadecanone (13.78), camphor (9.41), phytol (5.75) | not evaluated | [65] |
| E. hebecarpa Boiss. | Iran | Aerial parts | Hydro-distillation | α-Bisabolol (31.2), cis-cadin-4-en-7-ol (20.1), trans-piperitol (8.6), cis-p-menth-2-en-1-ol (6.4), trans-p-menth-2-en-1-ol (6.2) | not evaluated | [66] |
| E. helioscopia L. | Greece | Inflorescences | Steam distillation | Phytol (21.2), β-caryophyllene (10.0), behenic acid methyl ester (8.1), myristic acid methyl ester (5.5) | not evaluated | [59] |
| E. helioscopia L. | Turkey | Aerial parts | Hydro-distillation | β-Cubebene (19.3), palmitic acid (12.2), caryophyllene oxide (11.7), τ-elemene (9.3), spathulenol (9.3), phytol (6.9), hexahydrofarnesly acetone (5.3) | Low antioxidant and antiacetylcholinesterase activity, moderate butyrylcholinesterase and similar anti-urease activity to thiourea. | [67] |
| E. heterophylla L. | Nigeria | Leaves | Hydro-distillation | 3,7,12,15-Tetramethyl-2-hexadecen-1-ol (12.30), stearic acid (11.21), oleic acid (10.42), linoleic acid (8.97), 1,2-epoxy-cyclododecane (7.91), 13-tetradece-11-yn-1-ol (7.83), 7,10-hexadecadienal (7.62), 1,2,15,16-diepoxyhexadecane (6.37), phytol (6.32), 2-monopalmitin (5.43) | Toxic to brine shrimp larvae (LC50 = 21.7 µg/mL). Radical scavenging activity similar to ascorbic acid, lower than BHA but higher than α-tocopherol at 250 µg/mL. | [68] |
| E. heterophylla L. | Nigeria | Stems | Hydro-distillation | Stearic acid (11.21), oleic acid (10.42), linoleic acid (8.97), 1,2-epoxy-cyclododecane (7.91), 13-tetradece-11-yn-1-ol (7.83), 7,10-hexadecadienal (7.62), 1,2,15,16-diepoxyhexadecane (6.37), phytol (6.32), 2-monopalmitin (5.43), 2-aminoethoxyethynediyl methyl ester (5.40) | Very toxic to brine shrimp larvae (LC50 = 8.94 µg/mL). Radical scavenging activity similar to ascorbic acid, lower than BHA but higher than α-tocopherol at 250 µg/mL. | [68] |
| E. heterophylla L. | Egypt | Aerial parts | Hydro-distillation | 1,8-Cineole (32.0), camphor (16.5), β-elemene (5.9 ) | Radical scavenging activity (IC50 325.3 µL/L) lower than ascorbic acid (204.4 µL/L). | [69] |
| E. hirta L. | Lagos | Leaves | Hydro-distillation | Phytol and its isomeric forms (34.8), 6,10,14-trimethyl-2-pentadecanone (12.37), hexadecanal (7.63), palmitic acid (6.26) | not evaluated | [70] |
| E. macroclada Boiss. | Turkey | Aerial parts | Hydro-distillation | Tetratetracontane (42.7), hexatriacontane (12), mint furanone (6.0) | Anti-lipid peroxidation activity (IC50 = 14.8 µg/mL) similar to α-tocopherol. Lower radical scavenging activity than BHT but higher than E. gaillardotii essential oil. | [64] |
| E. macrorrhiza C.A.Mey. ex Ledeb. | China | Aerial parts | Hydro-distillation | Acorenone B (16.72), (+)-cycloisosativene (14.94), 3β-hydroxy-5α-androstane (10.62), β-cedrene (8.40), copaene (7.37), palmitic acid (5.68) | Cytotoxic activity against Caco-2 cell line (IC50 = 78.32 µg/mL), antibacterial activity against Staphyloccocus aureus (MIC = 5.6 µg/mL) but lower than ampicillin (MIC = 0.25 µg/mL) | [71] |
| E. macrorrhiza C.A.Mey. ex Ledeb. | China | Roots | Hydro-distillation | Acorenone B (25.80), (+)-cycloisosativene (12.40), β-cedrene (7.98), copaene (6.29), 3β-hydroxy-5α-androstane (5.52) | Cytotoxic activity against Caco-2 cell line (IC50 = 11.86 µg/mL), antibacterial activity against Staphyloccocus aureus (MIC = 2.8 µg/mL) but lower than ampicillin (MIC = 0.25 µg/mL) | [71] |
| E. pekinensis Rupr. | China | Roots | Steam distillation | Agarospirol (49.23), hedycargol (20.66) | not evaluated | [72] |
| E. pilosa L. | India | Aerial parts | Hydro-distillation | Phytol (5.75), n-pentadecanal (5.12) | not evaluated | [73] |
| E. rigida M.Bieb. | Greece | Inflorescences | Steam distillation | Heneicosane (13.8), heptacosane (12.7), β-caryophyllene (9.4), linalool (6.7), pentacosane (6.5) | not evaluated | [59] |
| E. sanctae-caterinae Fayed | Egypt | Aerial parts | Hydro-distillation | Valencene (16.01), (+) spathulenol (15.41), (-)-caryophyllene oxide (10.50), limonene (7.66) | not evaluated | [74] |
| E. sanctae-caterinae Fayed | Egypt | Aerial parts | Microwave-assisted | Butyl hydroxyl toluene (25.58), β-eudesmol (13.67), 6-epi-shyobunol (11.83), (+) spathulenol (10.32), thymol (7.00) | not evaluated | [74] |
| E. teheranica Boiss. | Iran | Aerial parts | Hydro-distillation | Elemol (57.5), β-caryophyllene (8.1%), caryophyllene oxide (7.8%) | not evaluated | [75] |
| E. thymifolia L. | India | Aerial parts | Steam distillation | Palmitic acid (33.03), phytol (10.367), myristic acid (6.58) | not evaluated | [76] |
| E. tithymaloides L. | Bangladesh | Aerial parts | Steam distillation | Eugenol (22.52), phenyl ethyl alcohol (14.63), 3-pentanol (9.22), caryophyllene oxide (7.73), isoeugenol (7.32), pentadecanol (5.14), spathulenol (5.11) | Radical scavenging activity (DPPH IC50 = 13.67 µg/mL) higher than BHA (IC50 = 18.26 µg/mL). | [77] |