2. Bacteria of the Genus Bacillus in the Biodegradation of Some Synthetic Plastics
Bacteria of the genus
Bacillus biodegrade polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), poly (ethylene terephthalate) (PET) and polyurethane (PUR). These polymers are synthesized from fossil hydrocarbons, a non-renewable resource, and comprise more than 90% of the global total production of polymeric materials
[8]. Enzymes released by microorganisms during growth and development act as aggressive media in biodamage of insulating materials
[1]. In particular, such compounds are able to produce the bacterium
B. mesentericus (
B. subtilis), found on polymer coatings
[1]. Degradation of polyethylene can be classified as abiotic (caused by temperature and UV irradiation) or biotic (biodegradation caused by the action of microorganisms). Microorganisms modify and consume the polymer, leading to changes in its properties. Bacteria of the genus
Bacillus, among other bacterial strains, are involved in the biodegradation of polyethylene
[9][10].
B. amyloliquefaciens, B. brevis, B. cereus, B. circulans, B. halodenitrificans, B. mycoides, B. pumilus, B. sphericus and
B. thuringiensis appear to be particularly active agents causing biodegradation of this material. Restrepo-Flórez et al.
[9] highlight that although the damage to polyethylene is connected to only one of these two damage modes, both typically act cooperatively in nature
[11]. The abiotic mechanisms of deterioration of polyethylene have been described in
[11]. The biodegradation of polyethylene and mechanisms associated with this process are analyzed in review publications
[9][10]. The biodegradation of polyethylene under normal conditions is extremely slow, while the knowledge of complete metabolic pathways involved in the process, as well as the inventory and structure of respective enzymes, is quite basic
[9]. The authors discuss changes of PE in the presence of microorganisms: (1) functional groups on the surface; (2) the surface topography; (3) the hydrophobicity/hydrophilicity of a surface; (4) mechanical properties; (5) crystallinity; (6) molecular weight distribution. It is indicated that the loss of molecular weight and the oxidation of the molecules are the result of synergistic effects caused by biotic and abiotic factors (photo-oxidation or heat treatment)
[9]. In particular, thermo-irradiation pretreatment was important for the biodegradation of PE by
Bacillus amyloliquefaciens (B. amyloliquefaciens) [12]. Biofilm colonization is the initial step in the degradation of this polymer
[9]. Bacteria that form biofilms are capable of producing surfactants, molecules that can mediate the attachment process of microorganisms to a hydrophobic surface
[9]. Hydrocarbon polymers are resistant to biodegradation, which results from highly stable C-C and C-H covalent bonds
[13]. Restrepo-Flórez et al.
[9] note the theoretical possibility of polyethylene being used as a carbon source for microorganisms, similar to many other hydrocarbons, but subject to the reduction in PE molecular weight by the chemical/biochemical processes. The presence, in some members of the genus
Bacillus, of enzymes related to the
Pseudomonas putida GPo1 membrane-bound alkane hydroxylase system
[14] increases this probability. Such enzymes are involved in a complicated chemical reaction: the regio- and stereospecific activation of C-H bonds. There are reports that the weight loss of degradable polyethylene (PE with chemical or photoinitiators or both) positively correlated with CO
2 emission in the consortium of
Pseudomonas frequentans +
B. mycoides under the condition of treatments with or without preheating
[15]. Based on this, the researchers conclude that the disposal of degradable polyethylene by these bacteria is a source of carbon.
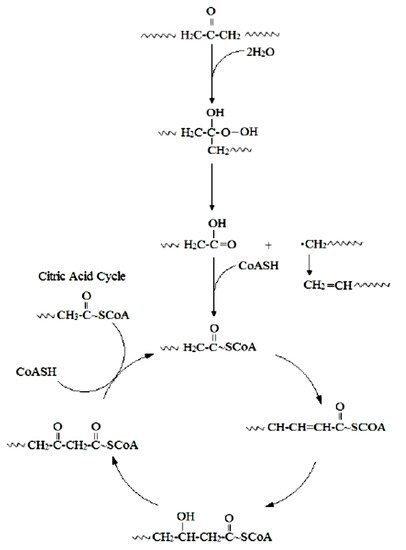
The initial step involves hydroxylation of C-C bonds, generating primary or secondary alcohols, which are further oxidized to aldehydes or ketones, and then to carboxylic acids
[16]. Thus, microbial oxidation decreases the number of carbonyl groups due to the formation of carboxylic acids. Carboxylated n-alkanes are analogous to fatty acids, which can be catabolized by bacteria via the β-oxidation system pathway (
Figure 1). However, neither the breaking of C-C bonds within the backbone of PE polymers nor the generation of long carbon chain carboxylic acid hydrolysis products have been reported
[16].
Figure 1. Mechanism for the biodegradation of PE
[16].
Thus, bacterial isolates
B. amyloliquefaciens contributed to the depolymerization of biodegraded products in extracellular media, indicating the biodegradation process of PE
[17]. The participation of
B. brevis in PE biodegradation is noted in the work of Wasserbauer et al.
[18].
B. cereus degraded UV-irradiated PE associated with a pronounced extracellular production of both laccases and MnP
[19]. Biodegradable properties against PE were also observed for
B. cereus strain Ma-Su CECRI-1
[20] and
B. cereus strain A5,a
[21]. In the latter case, a high biodegradable activity (35.72 ± 4.01%) in relation to PE was registered
[21]. The ability to biodegrade PE is also inherent to
B. subtilis [22].
B. subtilis produced surface active compounds (biosurfactants) that enhance the degradation process of PE
[23].
The ability of
B. mycoides and
B. subtilis (
Bacillus species indigenous to the Niger Delta mangrove soil) to biodegrade polyethylene was studied by Ibiene et al.
[24].
Bacillus safensis and
B. amyloliquefaciens have demonstrated the biodegradable ability for low-density polyethylene thermoplastic. Moreover,
B. safensis had better biodegradable ability than
B. amyloliquefaciens, shown by the registered weight reduction
[25].
It is worth noting that only genus (
Bacillus) identification of bacteria has been established in most studies. Thus, in a number of studies,
Bacillus sp. are indicated as polyethylene-degrading bacteria
[26][27][28][29][30][31], the ability of which to form a biofilm on the surface of this material and reduce its hydrophobicity by isolated bacteria was noted in the article of Yang et al.
[29]. The biofilm formation on the surface of polyethylene and its biodegradation were also observed for
B. mycoides [15].
Polypropylene was biodegraded by the
B. flexus + Pseudomonas azotoformans and
B. flexus + B. subtilis associations
[32]. Polypropylene was subjected to biodegradation by
B. cereus [33][34],
B. thuringenesis and
B. licheniformis [34]. The latter three species are also involved in the biodegradation of polypropylene-poly-L-lactide
[34]. Degradation of PP microplastics by
B. gottheilii caused a weight loss of 3.6% after 40 days
[35].
Biodegradation of polypropylene has been improved by using polymer blends of carbohydrates, starch or cellulose blends such as that reported for polyethylene and polystyrene
[36]. The use of the blends facilitates adhesion of the microorganisms to the surface of the polymer and acts as a co-metabolite. Biodegradation of polycaprolactone blended PP has also been demonstrated using lipase since lipase is well known to degrade the ester linkages of polycaprolactone
[36].
Bacillus sp. degrades polystyrene
[37][38][39]. The following studies provide insights into biodegradation of PS caused by
B. aryabhattai [40],
B. subtilis [41], and
B. paralicheniformis [42].
B. cereus and
B. gottheilii reduced PS granule weight by 7.4% and 5.8%, respectively, within 40 days
[35].
B. cereus was also noted as a biodegradable PS in
[43].
Strains
B. subtilis and
B. cereus formed biofilm on PET and poly (lactic acid), demonstrating biochemical activity and accelerating biodegradation of both plastic materials
[44].
B. cereus was also identified as part of two consortia capable of biofilm formation and PET degradation
[45].
B. megaterium formed a biofilm on PET film
[8]. Carboxylesterases from
B. licheniformis and
B. subtilis partially hydrolyzed PET fibers and showed a high activity against PET oligomers
[46].
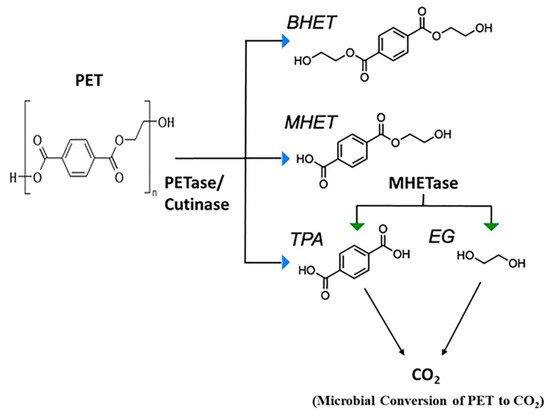
The mechanism of PET biodegradation is demonstrated in
Figure 2. The PET-digesting enzyme labeled as PETase converts PET to mono(2-hydroxyethyl) terephthalic acid (MHET), with minimal amounts of terephthalic acid (TPA) and bis(2-hydroxyethyl)-TPA as secondary products
[36].
Figure 2. Microbial degradation of PET
[36].
PETase, polyethylene terephthalate (PET) hydrolase or PET-digesting enzyme;
BHET, bis(2-hydroxyethyl) terephthalic acid;
MHET, mono(2-hydroxyethyl) terephthalic acid;
TPA, terephthalic acid;
EG, ethylene glycol.
B. flexus has been proved to biodegrade poly (vinyl chloride) (PVC) film
[47]. Bacteria of this species have been able to form dense biofilm on the plastic film surface and cause a decrease in the mean molecular weight of the PVC film
[47].
Bacillus sp., triggering degradation of PVC, caused weight loss of the material of 0.26 ± 0.02% in comparison to initial weights after 90 days
[31].
The involvement of bacteria of the genus
Bacillus in the biodegradation of polyurethanes is discussed in a review article by G.T. Howard
[48]. PUR-degrading bacteria are
B. chitinolyticus and
B. pumilus [49] and
B. subtilis [50][51][52].
Oxidative degradation has been generally associated with poly(ether-urethane)
[53]. This mechanism is illustrated by the following reactions.
Thus, the reactive oxygen species initiate degradation of polyether urethanes through oxidative attack of the soft segment. This reactive oxygen species abstracts an alpha-methylene hydrogen atom from the polyether soft segment. The addition of a hydroxyl radical to the carbon radical forms a hemiacetal, which oxidizes to an ester. Acid hydrolysis of the ester results in chain scission of the soft segment and formation of acid end groups. Significant chain scission results in the solubilization and extraction of low molecular weight degradation products
[53].
A similar oxidative mechanism was proposed for hard segment degradation
[53]. Oxygen radicals abstract an alpha-methylene hydrogen atom from the chain extender at the urethane. Additional hydroxyl radicals combine with the chain radical to form a highly reactive carbonyl hemiacetal. Oxidative hydrolysis of the carbonyl hemiacetal results in chain scission and formation of an unstable carbamic acid and carboxylic acid end groups
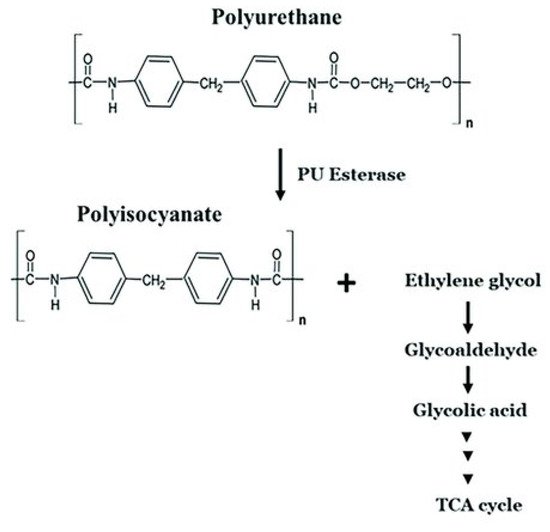
[53]. The carbamic acid decarboxylates readily to form a free amine. The mechanism of polyurethane biodegradation with the participation of polyurethane esterases is shown by Mohanan et al.
[36] (
Figure 3).
Figure 3. Microbial degradation pathway of polyurethane
[36]. TCA cycle: the cycle of tricarboxylic acids. PU esterase: polyurethane esterase.
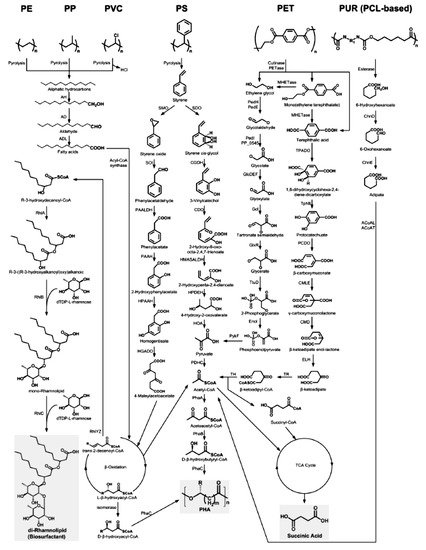
Ru et al.
[54] present the metabolic pathways of degradation of the plastics considered by us (
Figure 4).
Figure 4. The metabolic pathways of depolymerization products of six kinds of plastics
[54]. Plastics: PE, polyethylene; PS, polystyrene; PP, polypropylene; PVC, polyvinyl chloride; PUR, polyurethane; PCL, polycaprolactone diol; PET, polyethylene terephthalate. Enzymes: AH, alkane hydroxylase; AD, alcohol dehydrogenase; ALD, aldehyde dehydrogenase; RhlYZ, R-specific enoyl-CoA hydratase; RhlA, HAA synthetase; RhlB, rhamnosyltransferase 1; RhlC, rhamnosyltransferase 2; SMO, styrene monooxgenase; SOI styrene oxide isomerase; PAALDH, phenacetaldehyde dehydrogenase; PAAH, phenylacetate hydroxylase; HPAAH, 2-hydroxyphenylacetate hydroxylase; HGADO, homogentisate 1,2-dioxygenase; SDO, styrene dioxygenase; CGDH, cis-glycol dehydrogenase; CDO, catechol 2,3-dioxygenase; HMASALDH, 2-hydroxymuconic acid semialdehyde hydrolase; HPDEH, 2-hydroxypenta-2,4-dienoate hydratase; HOA, 4-hydroxy-2-oxovalerate aldolase; PDHC, pyruvate dehydrogenase complex; PhaA, b-ketothiolase; PhaB acetoacetyl-CoA reductase; PhaC, PHA synthase; PedH, quinoprotein alcohol dehydrogenase; PedE, quinoprotein alcohol dehydrogenase; PedI, aldehyde dehydrogenase family protein; PP_0545, aldehyde dehydrogenase family protein; GlcDEF, glycolate oxidase; Gcl glyoxylate carboligase; GlxR, tartronate semialdehyde reductase; TtuD, hydroxypyruvate reductase; PykF, pyruvate kinase; TPADO, TPA dioxygenase; TphB, 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylate dehydrogenase; PCDO, protocatechuate 3,4-dioxygenase; CMLE, b-carboxy-cis,cis-muconate lactonizing enzyme; CMD, b-carboxymuconolactone decarboxylase; ELH, enollactone hydrolase; TR, b-ketoadipate:succinyl-CoA transferase; TH, b-ketoadipyl-CoA thiolase; ChnD, 6-hydroxycaproate dehydrogenase; ChnE, 6-oxohexanoic dehydrogenase; ACoAL, adipate-CoA ligase; ACoAT, acetyl-CoA C-acyltransferase
[54].
3. The Application of Bacteria of the Genus Bacillus to Materials’ Biodamage Control
It should be noted that today the question of the application of biological control for protection against corrosion damage is debatable, as none of the methods for corrosion inhibition by microorganisms found to be successful reached the practical application stage
[7]. However, there are noteworthy publications on the possibility of using bacteria of the genus
Bacillus to control the biodamage of materials.
The inhibition of corrosion by bacteria is often accomplished by: (1) a decrease in the corrosive action of the medium in restricted parts of the metal–bulk solution interface, for instance, by neutralizing the acidity of the medium; (2) the formation of protective films on the metal surface or providing new protective films, in particular through the production of EPS with metal-binding abilities; and (3) decreasing the cathodic reaction due to the consumption of a cathodic electron acceptor by microbes
[6].
In addition, the biocontrol properties of some microorganisms are determined by their high antibacterial properties associated with the formation of antimicrobial compounds
[55] and antibiofilm-forming properties due to their ability to affect the quorum sensing system
[56]. Such microorganisms include members of the genus
Bacillus. Thus, a number of studies have proved that corrosion results from the formation of biofilms, while
Bacillus sp.
[57][58],
B. thuringiensis strain SN8
[59],
B. cereus [60],
B. licheniformis (including genetically modified ones)
[61],
B. subtilis [62][63][64],
B. firmus strain H2O-1
[65][66] and
B. brevis [62] are responsible for producing antimicrobial compounds.
In particular, in conditions of artificial seawater in the presence of a protective biofilm of
B. subtilis strain WB600, passivation of Al 2024 was recorded. The addition of antibiotics led to the death of bacteria in the biofilm, which was reflected in the impedance spectra. Adding antibiotics to sterile artificial seawater had no effect on corrosion processes
[64].
The inhibiting effect of
B. thuringiensis strain SN8 on steel is shown in the publication by Bano et al.
[59].
B. thuringiensis strain SN8 inhibited the MIC of materials in soil conditions over a period of 150 days. The corrosion-preventing properties of the
B. thuringiensis strain SN8 dissipated after this period. It stands to reason that corrosion-inhibiting/protective properties of biological agents must be verified in term of the maximum time frame for their effectiveness
[67].
The inhibitory effect of a biofilm formed by
B. cereus was shown by Aїmeur et al.
[60].
Ornek et al. evaluated the effect of aluminum-chelating anionic peptides, which release biofilms of natural and genetically modified
Bacillus on the pitting of aluminum alloy in continuous reactors
[61]. The corrosion rate of aluminum alloy 2024 was reduced by 90% due to γ-polyglutamate-forming biofilm of
B. licheniformis. Meanwhile, one of the peptides that secreted the genetically engineered biofilm of
B. subtilis only slightly reduced the pitting compared to the biofilm on the control material, which already significantly reduces the corrosion of aluminum compared to the sterile control one.
From thermophilic
B. licheniformis on a steel substrate, the bacterial biofilm and the nature of its extracellular polymeric substances have been probed chemically and electrochemically for their influences on metal dissolution within an incubation period
[68]. Corrosion inhibition in the presence of varying concentrations (in CFU/ml) of these bacteria in biotic inoculate systems is explained in terms of corrosion resistance and biofilm capacity. Steel’s corrosion rate is reduced significantly in saline culture medium within the range of concentrations of bacteria under study compared with the sterile control. This is attributed to the adhesion of relatively compact and dense “beneficial” biofilm as well as the secretion of corrosion-inhibiting substances from the bacterial biofilm as revealed during surface analysis
[68].
Jayaraman et al.
[62] found that biofilms of genetically engineered
B. subtilis secreting the antibiotics indolicidin, bactenecin and probacterin are able to inhibit the growth of SRB that cause corrosion (
D. vulgaris and
D. gigas) and significantly reduce corrosion in a continuous culture.
The gramicidin S peptide, which naturally produced the biofilm-forming strain of
B. brevis, also inhibited the colonization of SRB and reduced the corrosion of the steel
[69]. It should be noted that antibiotics, such as ampicillin, inhibited only the growth of SRB when added prior to the colonization of SRB. At the same time, the associative culture of
B. brevis (producing S-gramicidin) with SRB completely blocked the growth of the latter.
Bacillus sp. strain H2O-1, isolated from the connate water of a Brazilian reservoir, produces an antimicrobial substance (denoted as AMS H2O-1) that is active against sulfate-reducing bacteria, which are the major bacterial group responsible for biogenic souring and biocorrosion in petroleum reservoirs. AMS H2O-1 is a mixture of four surfactin-like homologues, and its biocidal activity and surfactant properties suggest that this compound may be a good candidate for sulfate-reducing bacterial control. Thus, it is a potential alternative to the chemical biocides or surface-coating agents currently used to prevent SRB growth in petroleum industries
[65].
A new antimicrobial substance is being developed by using biosurfactant produced by indigenous oil reservoir
Bacillus sp. to prevent and eradicate biofilm
[58]. The biosurfactant is able to inhibit
Pseudomonas sp. strain 1 and
Pseudomonas sp. strain 2 attachment to carbon steel ST37 surfaces and also able to eradicate preformed biofilm on steel surfaces. This study also showed the reduction of corrosion rate in carbon steel ST37 as a result of material treatment with biosurfactants. The authors suggest that the biosurfactant in this study could be a good candidate for a new anticorrosion agent
[58].
The effects of bacteria of the genus
Bacillus depend not only on the formation of antimicrobial compounds and protective biofilm, but also on the type of damaged material. In particular, Juzeliunas and co-authors found that
B. mycoides accelerates zinc corrosion, inhibits aluminum corrosion and does not affect mild steel
[70].
Bacillus sp. strain ПКИ-12Л is considered to be a promising biological agent for the creation of a biological product for the protection of polymeric composite materials from biological damage due to its fungicidal activity
[71].
The synthesis of extracellular molecules such as biosurfactants or proteins should have major influence on bacterial adhesion. These molecules may be adsorbed on surfaces and modify their energy characteristics. Under these conditions, adhesion rates are not dependent upon the initial characteristics of the substrate but on those of fouled surfaces
[72].
Lipopeptides produced by
B. subtilis (surfactin, iturin and fengycin) are able to modify the surface hydrophobicity of stainless steel and Teflon and, consequently, influence microbial adhesion to their surfaces
[73]. The authors showed that these effects depend on the lipopeptide type, the concentration and the substratum. Thus, iturin A had no effect in relation to stainless steel; surfactin and fengycin increased the hydrophobicity of steel. The bioconditioning of surfaces using microbial surfactants has been suggested as a new strategy for reducing adhesion of bacteria to surfaces
[73].
Among the representatives of the genus
Bacillus as controlling agents of biodamage, the bacterium
B. velezensis deserves attention
[56][55]. It should be noted that the current heterotypic synonyms of
B. velezensis are
B. amyloliquefaciens subsp.
plantarum,
B. methylotrophicus, “
B. oryzicola”, and
B. methylotrophicus subsp.
plantarum [74][75].
In the article of Wang et al.
[76], high activity against marine fouling by proteases isolated from
B. velezensis strain SM-1 was noted. This may be the basis of the modern eco-friendly approach to creating materials against fouling. Khan et al. isolated the bacterium
B. methylotrophicus WY with the ability to quench the quorum, which can be used to control membrane biofouling
[56]. In particular, it was discovered that this strain has significant influence on the degradation of acylhomoserinlactone (AHSL)—a kind of signaling molecule required for the development of biofilm. The inhibitory activity of
B. velezensis strain K68 against the biofilm of
Streptococcus mutans on polystyrene was studied
[77].
Bacillus sp. strain H2O-1 (with 99.8 and 99.5% similarity of the 16S rRNA gene sequence between it and the type strains of
B. amyloliquefaciens and
B. methylotrophicus, respectively) produced an antimicrobial substance that was active against sulfate-reducing bacteria
[65].
High concentrations of the catalase and peroxidase enzymes produced by some strains of
Bacillus sp. can inhibit corrosion
[78]. However, the exact mechanism and enzymatic role are unknown. Further research targets the potential role of these enzymes
[78].