1000/1000
Hot
Most Recent

Two-dimensional (2D) materials are generally defined as crystalline substances with a few atoms thickness.Two-dimensional transition metal dichalcogenide (2D-TMDs) semiconducting (SC) materials have exhibited unique optical and electrical properties. The layered configuration of the 2D-TMDs materials is at the origin of their strong interaction with light and the relatively high mobility of their charge carriers, which in turn prompted their use in many optoelectronic applications, such as ultra-thin field-effect transistors, photo-detectors, light emitting diode, and solar-cells. Generally, 2D-TMDs form a family of graphite-like layered thin semiconducting structures with the chemical formula of MX2, where M refers to a transition metal atom (Mo, W, etc.) and X is a chalcogen atom (Se, S, etc.). The layered nature of this class of 2D materials induces a strong anisotropy in their electrical, chemical, mechanical, and thermal properties. In particular, molybdenum disulfide (MoS2) is the most studied layered 2D-TMD.
From a crystalline point of view, layered MoS2 exists in three polymorphic crystalline structures: 1T (tetragonal) [1], 2H (hexagonal) [2], and 3R (rhombohedral) [3] (Figure 1). The crystallographic parameters associated to these crystalline forms are summarized in Table 1. In the case of mono- to few-layer structures, 2H-MoS2 is the most thermodynamically stable phase and thus the most commonly encountered. When the MoS2 is in the monolayer form, it takes an octahedral or a trigonal prismatic coordination phase.
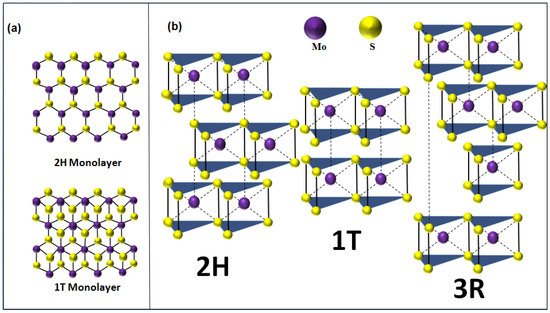
Figure 1. (a) Top view of 2H/1T MoS2 monolayer. (b) Polymorphic structures of MoS2 (2H is the hexagonal crystal form, 1T is the tetragonal crystal form, and 3R is the rhombohedral crystal form).
Table 1. Crystal parameters and the nature of polymorphic structures of 2D-MoS2.
Furthermore, MoS2 layered materials were observed to exhibit various shapes and morphologies, such as planar [4][5][6] and vertically aligned nanosheets (NSs) [7], nanoflowers [8], nanotubes [9], nanowires [10], and nanoplatelets [11][12]. This variety of forms could be controlled by choosing suitable synthesis routes with optimized operating parameters [8][9][10][11][13][14][15][16][17]. Thus, it is possible to adjust the 2D-MoS2 properties to develop high performance devices i energy storage [17], electronics [16], photonics [15], sensing [18], and field emission [19] applications. Recently, up to few-layer MoS2 nanosheets have been shown to be highly efficient for electronic, optoelectronic, and solar energy harvesting devices [20][21][22] because of their tunable direct bandgap [23], strong light-absorption, and prominent photoluminescence with energies lying in the visible range (1.8–1.9 eV) [24].
Although Mo and S are strongly covalently bonded within an individual layer, adjacent sheets are linked together only by the very weak van der Waals interaction. This weak bonding provides a facile processing route such as mechanical or chemical exfoliation to form few- to monolayer MoS2 films. Unlike graphene, 2D-MoS2 is much less prone to surface contaminations, which offers a superior chemical stability to 2D-MoS2, making it more attractive for the above-mentioned applications [25][26][27].
Tremendous efforts have been devoted to the synthesis of 2D-MoS2 with controllable large-area growth and uniform atomic layers using both top-down and bottom-up approaches. The most commonly used processing routes are detailed in the following sub-sections along with their advantages and limitations.
Mechanical exfoliation, also known as micromechanical cleavage, is a straightforward technique that takes advantage of the weak bonding between layers, for the production of high-quality mono- to few-layer MoS2 [28][29][30]. It consists of exfoliating thin films of 2D-MoS2 from a bulk MoS2 crystal by using a low surface tension tape to break the weak interlayer bonds in a similar way as for grapheme [31]. Additional exfoliation of the extracted films may be needed to obtain few- to monolayer MoS2. Tapes could be attached to glass slides to achieve planar exfoliation and slow peeling. The obtained monolayers are usually transferred to an appropriate substrate for further analysis and testing.
The advantage of the mechanical exfoliation process lies in its simplicity that requires the sole use of a confocal microscope to localize the 2D-MoS2 layers deposited on the substrate. Conveniently, this technique can produce high crystalline quality mono- to few layers with a lateral size up to few tens of micrometers, making them highly suitable for sensing applications. However, this approach suffers from a lack of a consistent control in producing the 2D monolayers as it is heavily user-dependent and does not permit the control of the size and/or thickness uniformity of the exfoliated 2D-MoS2 layers [32]. Therefore, the mechanical exfoliation technique is not necessarily suitable for the production of 2D-MoS2 layers intended for large-area and high-throughput applications.
Chemical exfoliation, on the other hand, appears as a promising approach to produce large quantities of mono- and few-layer MoS2 nanosheets [30][33][34][35]. Eda et al. [24] reported a high yield of monolayer crystal synthesis using chemical exfoliation of bulk MoS2 via Li intercalation. However, this approach may induce an alteration in the quality of the produced 2D-MoS2. For instance, the chemically exfoliated MoS2 layers can lose their semiconducting properties because of the structural changes resulting from the Li intercalation process. However, this fabrication route stands by its ease of processing, low production costs, and suitability for catalysis and/or sensing applications [36].
Chemical vapor deposition (CVD) is one of the most popular routes for large-scale, high-quality, and low-cost 2D-MoS2 material production [19][37][38][39]. CVD is a bottom-up fabrication method at the equilibrium state, which enables the processing of layered 2D-MoS2 with controlled morphology and good crystallinity while minimizing structural defects. The control of the CVD process is ensured by tuning the deposition parameters such as temperature, pressure, gas flow rate, precursor’s quantities, and substrate types. The 2D-MoS2 synthesis via the CVD technique can be achieved by means of thermal vapor sulfurization (TVS), thermal vapor deposition (TVD), and thermal decomposition (TD). Deokar et al. [13] used TVS for high quality and vertically-aligned luminescent MoS2 nanosheets. A similar process could be used to grow 2D-MoS2 layers [6][40] by employing two sources, such as molybdenum thin film (below 20 nm) or molybdenum oxide (MoO3) powder deposited on a SiO2/Si substrate as a first precursor and the sulfur powder or gaseous sulfur source (H2S, etc.) as the second precursor [19][37][38][39][41][42]. A typical CVD sulfurization process (Figure 2a) is usually performed in a tubular furnace reactor, where a continuous argon flow (typical flow rate 100 sccm) is used as a carrier gas to stream the evaporated sulfur into the Mo source materials.
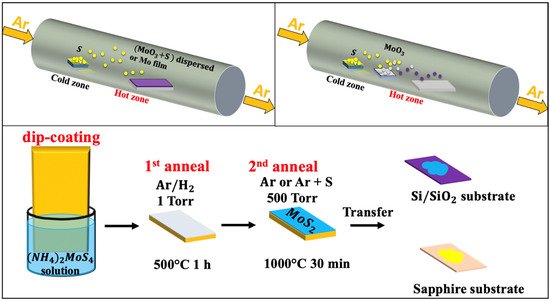
Figure 2. Schematic of the chemical vapor deposition techniques: (a) thermal vapor sulfurization process using a quartz tube; (b) thermal vapor deposition process using a quartz tube; and (c) thermal decomposition of (NH4)2MoS4 (reproduced and adapted from Ref. [4]).
One of the critical aspects to be controlled in such a CVD tubular reactor is the temperature gradient between the S powder and the substrate. In fact, while the S powder is at 150–200 °C, the substrate’s temperature—with or without Mo thin film—should be maintained in the 700–900 °C range to obtain the 2D-MoS2 phase. This technique offers sufficient latitude to fairly control the thickness and the homogeneity of the grown 2D-MoS2. The typical average lateral crystal size obtained by CVD is usually in the 10–30 nm range. Table 2 shows few examples of CVD-TVS grown MoS2 nanostructures along with their associated processing conditions.
Table 2. Examples of CVD-TVS grown MoS2 nanostructures.
| Substrate | Precursors | Growth Conditions | Morphology | Ref |
|---|---|---|---|---|
| Si | MoO3 and S powders dispersed on substrate | MoO3 and S powders dispersed on substrate at 850 °C; S powder at 400 °C; Ar-0.725 L/min; time reaction = 30 min | MoS2 nanosheets | [13] |
| Si [001] | S powder and Mo film deposited on substrate | Mo deposited on Silicon at 850 °C, S at 400 °C; Ar-0.725 L/min; time reaction = 30 min | MoS2 nanosheets | [14] |
| Si/SiO2 | S powder and Mo film deposited on substrate | Mo deposited on Silicon at 850 °C, S at 400 °C; Ar-0.725 L/min; time reaction = 30 min | MoS2 nanosheets | [19] |
| Diamond substrate | S powder and Mo deposited on substrate | Mo deposited on Silicon with S powder at 800 °C; N2; ambient pressure; time reaction = 30 min | Horizontally and vertically MoS2 | [43] |
| Si/SiO2 | S powder and MoO3 deposited on substrate | MoO3 film deposited on Silicon at 750–850 °C, 600 mg of S powder at 100 °C; Ar-0.01 L/min; time reaction = 10 min | Mono-to few-layers of MoS2 | [44] |
Table 2 shows the typical morphologies obtained for MoS2, which seem to depend on the carrier gas and the type of the substrate used. The reaction time and the spatial position of the substrate strongly affect the number of resulting layers.
The TVD based MoS2 growth (Figure 2b) involves the concomitant evaporation of both MoO3 and S powders. This approach consists of a stepwise sulfurization of MoO3 to form the MoS2 phase. It has been shown that, by increasing the S vapor flux, the sulfurization proceeds through several phase changes before reaching the final product. First, MoO3 is formed, then MoO2 followed by MoOS2, and finally MoS2. This approach is very useful to obtain 2D MoS2 layers with a lateral size of few tens of microns. The TVD growth conditions of MoS2 under various conditions and with different characteristics are summarized in Table 3.
Table 3. Examples of TVD grown MoS2 along with their relevant processing conditions (* D is the distance between the MoO3 and S powders inside the tubular furnace).
| Substrate/Setup | MoO3 (mg) | S (mg) | D * (cm) | Gas, Flow (sccm) | T (°C), Time (min) | Morphology | Ref |
|---|---|---|---|---|---|---|---|
| Si face-down | 15 | 80 | 18 | Ar 10 to 500 |
700, 30 | Flake size between 5.1–47.9 µm | [45] |
| SiO2/Si face-up |
10 | 200 | 30 | Ar, 100 | 850, 20 | Monolayer, bilayer and trilayer MoS2 | [46] |
| SiO2/Si face-down |
10 | 100 | – | N2, 20 | 650, 20 | MoS2 monolayer | [47] |
| SiO2/Si face-down |
10-30 | – | 25 | Ar, 150 | 800, 10 | MoS2 triangular flakes | [48] |
| SiO2/Si face-up |
50 | 175 | – | N2, 300 | 750, 15 | MoS2 monolayer with lateral size of 50 µm | [49] |
In comparison to the results obtained by CVD-TVS summarized in Table 2, TVD exhibits high-yield fabrication of 2D-MoS2 monolayers generally exhibiting a triangular flakes shape. Besides, one can notice the two possible configurations of the substrate of interest in TVD face-up and face-down compared to CVD-TVS [45][46][47][48][49].
Moreover, the TD-based CVD method presents an alternative approach to produce highly crystalline MoS2 thin layers with superior electrical properties on insulating substrates [4]. Typically, the TD-CVD is based on the high-temperature annealing of a thermally decomposed ammonium thiomolybdate layer (NH4)2MoS4 in the presence of S, as illustrated in Figure 2c. It is worth noting that the excess in sulfur introduces changes in the shape, size, and morphology of fabricated MoS2. It also leads to a p-type MoS2 semiconductor by increasing the electrons deficiency. In contrast, the presence of sulfur vacancies in MoS2 was reported to have a direct impact on the catalytic properties of MoS2, suggesting a carriers’ mobility alteration [50]
Besides, the addition of S during the high-temperature annealing drastically enhances the crystallinity of MoS2. Relatively, centimeter-sized MoS2 crystals could be formed on Al2O3 substrates compared to SiO2 ones [5]. The fully covered Al2O3 substrate with an epitaxial monolayer of MoS2 was achieved at 930 °C. The MoS2 crystals nucleate in a single domain to pursue by domain-to-domain stitching process occurring during annealing at 1000 °C mediated by the oxygen flow. The difference in the self-limited monolayer growth observed between the SiO2 and Al2O3 substrates is related to the absorption energy barrier on MoS2 [7]. In particular, the growth of MoS2 on Al2O3 obeys the surface-limited epitaxial growth mode, which is not the case for the SiO2 due to lattice mismatch. Moreover, the patterning of the as-grown MoS2 layers has been reported by means of the polydimethylsiloxane (PDMS) stamps and the reuse of the substrate after transferring the MoS2 layers [5]. Recently, the epitaxial growth of centimeter wafer-scale single-crystal MoS2 monolayers on vicinal Au (111) thin films were also obtained at a processing temperature of 720 °C, by melting and re-solidifying commercial Au foils [6]. This allows overcoming the evolution of antiparallel domains and twin boundaries, leading to the formation of polycrystalline films. It has been proposed that the step edge of Au (111) induced the unidirectional nucleation, growth, and subsequent merging of MoS2 monolayer domains into single-crystalline films.
The atomic layer deposition (ALD) technique is known to produce high-quality thin films even at low temperatures, typically between 150 and 350 °C. Since ALD is an atom stepwise growth process, where the reactants are alternately injected into the growth area, it allows the purging of excess species and by-products after each reaction. As a result, high-quality films are obtained by sequential surface reactions. A schematic representation of the ALD synthesis of 2D-MoS2 can be found elsewhere [51].
Despite the challenges related to its synthesis conditions, ALD makes it possible to deposit crystalline MoS2 thin films at a relatively low temperature (<350 °C) followed by annealing. For instance, L.K. Tan et al. [52] reported the possibility to use ALD for the synthesis of highly crystallized MoS2 films on sapphire substrates at 300 °C. They prepared MoS2 films by alternating exposure of the substrate to Mo(V) chlorides (MoCl5) and hydrogen disulfide (H2S) vapors. Similarly, Mattinen et al. [53] proposed the use of a Mo based precursor, namely Mo(thd)3 (thd = 2,2,6,6 tetramethylheptane 3,5-dionato), with H2S as a sulfur source. They have been able to achieve a self-limiting growth and a linear film thickness control (with a very low growth rate of ≈0.025 Å per cycle). While the crystallinity of these MoS2 films was found to be particularly good (taking into account that the deposition was done at a low temperature), their surface was rather rough, consisting of flake-like grains with a size of ≈10–30 nm. One of the advantages of this process is the possibility to deposit layered MoS2 films on various substrates. Table 4 summarizes the main processing conditions used by different groups along with the achieved MoS2 film thicknesses.
Table 4. Summary of the ALD deposition conditions and achieved MoS2 film thicknesses.
| Substrate | Precursors | P (Torr) | T (°C) | Cycles | Thickness | Ref |
|---|---|---|---|---|---|---|
| SiO2/Si | Mo hexacarbonyl and dimethyldisulfide | 1.4–3.3 | 100 | 100 | ≈11 nm | [54] |
| SiO2/n-Si | MoCl5 and H2S | 0.75 | 350–450 | 100 | ≈9 nm | [55] |
| Al2O3 | Mo(NMe2)4 and H2S | – | 60 | 100 | ≈12 nm | [51] |
| Al2O3 2-inch wafer | MoCl5 and H2S | 0.001 | 300 | 50 | ≈9 nm | [52] |
| SiO2/Si | Mo(thd)3 (thd = 2,2,6,6 tetramethylheptane 3,5-dionato) and H2S | 3.75 | 300 | 100 | ≈25 nm | [53] |
| Al2O3 c-plane |
MoCl5 and hexamethyldisilathiane | 3.75 | 350 | 250 | ≈22 nm | [56] |
| Carbon nanotubes, Si-wafers and glass | bis(tbutylimino)bis(dimethylamino) Mo (VI) and H2S | 300 | 100–250 | 100 | ≈11 nm | [57] |
| Si, SiO2, Al2O3 | MoCl5 and H2S | 3.75 | 430–480 | 1 | 1 layer | [58] |
| Si | MoCl5 and H2S | – | 390–480 | 100 | ≈21.5 nm | [59] |
| SiO2 | Mo hexacarbonyl and H2S | – | 175 | 100 | ≈5 nm | [60] |
The ALD appears as a potentially interesting technique for the production of high-quality MoS2 ultrathin films at relatively low temperatures and with the ability to achieve excellent step coverage onto different substrates. However, the very low throughput of the ALD might hinder its scalability and competitiveness in comparison with other physical and/or chemical deposition methods.
Pulsed laser deposition (PLD) has emerged as one of the most promising physical vapor deposition (PVD) techniques for the deposition of MoS2 thin films. The PLD approach consists of shining a focused high-power laser beam onto the surface of a solid target to be ablated and deposited as a film on a substrate. PLD is a non-equilibrium process that leads to the absorption of very-short (15–20 ns) and highly-energetic laser pulses by the target and to the formation of a directive plasma plume. The laser-ablated species that form the plasma plume condense onto the substrate, leading to the growth of a thin film. The PLD is well known for its large process latitude, high-flexibility, and excellent process controllability. For instance, by controlling the number of laser ablation pulses and/or the background gas pressure, nanoparticles, and/or films with thicknesses varying from few nm to few microns can be synthesized. Figure 3 shows a schematic representation of a PLD system.
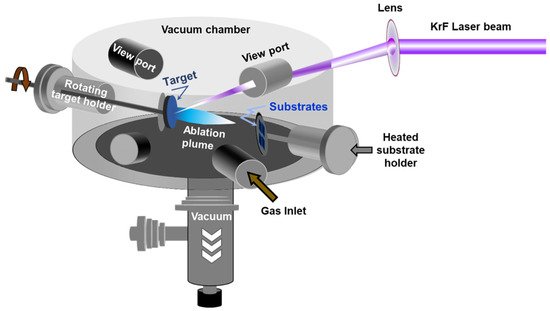
Figure 3. Schematic of the pulsed laser deposition chamber.
Among the advantages and the unique features of the PLD method, we can cite: (i) its ability to achieve a congruent transfer to the films when a multi-element target is used [61]; (ii) its highest instantaneous deposition rate along with the highly-energetic aspect of the ablated species (~10 times higher than in sputtering) enables the growth of metastable phases and/or crystalline phases even at room temperature; and (iii) its process latitude, which makes it easy to control almost independently each of the deposition parameters (laser intensity, number of laser ablation pulses, background gas pressure, and substrate temperature), and hence the properties of the deposited materials [62][63][64]. While the early studies on the PLD of MoS2 date back to the 1990s [65][66][67][68][69][70], it is only recently that important advancements have been made in PLD synthesis of 2D-MoS2 films onto various substrates opening thereby the way to their use for different optoelectronic applications. In 2014, PLD was successfully used to grow one to several layers of MoS2 onto different metal, semiconducting, and sapphire substrates [71][72]. Siegel et al. [73] were the first to report, in 2015, the growth of MoS2 films (from 1 to a few 10s of monolayers thick) on centimeter-sized areas. Other attempts were made to deposit ultrathin (≤3 nm) films of nearly-stoichiometric amorphous MoS2 onto irregular surfaces such as silicon and tungsten tips and to study their field electron emission (FEE) properties [65]. The authors stated that the addition of the MoS2 coating is beneficial to the FEE process since lower electric fields were required to extract an electron current density of 10 μA/cm2 (namely, 2.8 V/μm for MoS2-coated Si and ~5.5 V/μm for MoS2-coated W tips). More recently, PLD has been used to fabricate high-quality MoS2 films (monolayer to few layers) and integrated them into functional ultraviolet (UV) photodetectors [74]. The developed photodetectors were found to exhibit a very low dark current (~10 × 10−10 A), low operating voltage (2 V), and good response time (32 ms). Their performance surpassed that previously reported for 2D-MoS2 synthesized by other routes [75][76][77][78][79]. Indeed, under UV irradiation, their detectivity, photoresponse (Ion/Ioff ratio), and responsivity were found to be as high as 1.81 × 1014 Jones, 1.37 × 105, and 3 × 104 A/W, respectively. Table 5 summarizes most of the papers reported so far on the PLD of MoS2 films. More specifically, it compares the main PLD growth conditions of 2D-MoS2 films along with the obtained crystallographic phase and some of the reported optoelectronic properties.
Table 5. Summary of the PLD conditions of MoS2 films along with their thickness and some of their properties.
| Substrate | Target | P(Pa) | T(°C) | Laser Energy | Thickness | Properties | Ref |
|---|---|---|---|---|---|---|---|
| Stainless steel | MoS2 | 2.66 × 10−6 | RT/200/300/450 | 5 mJ | ≈400 nm | Granular structure stoichiometric, crystalline MoS2 | [80] |
| Stainless steel | MoS2 | 10−6 | RT/300 | 100 mJ | ≈70 nm | Stoichiometric single crystal MoS2 | [81] |
| c-Al2O3 (0001) and Si/SiO2 | 2H-MoS2 | 9.33 × 10−4 | 600 | 500 mJ/cm2 | ≈1.4 nm | Stoichiometric 2H phase Flake size ≈ 10 µm |
[82] |
| GaN/c-Al2O3 (0001) | 2H-MoS2 | 8 × 10−4 | 700 | 50 mJ | Few layers | Mixed phase Roughness ≈0.11 nm |
[72] |
| Titanium foil | p-MoS2 | 1.33 × 10−2 | RT | – | 0.65 nm | 1T phase MoS2 | [83] |
| SiO2 on Si [70] | MoS2 | 1.33 × 10−2 | 800 | 200 mJ/cm2 | ≈20–60 nm | 2H phase MoS2 | [74] |
| Gold-coated carbon cloth | Amorphous MoS2 | 1.33 × 10−2 | RT | 220 mJ/cm2 | ≈200 nm | 2H phase MoS2 | [67] |
| Quartz | MoS2 | 9 × 10−5 | 300 | 8500 mJ/cm2 | 30 layers | Mixed phase | [84] |
| Al2O3 (0001) | MoS2+S Powder |
1.33 × 10−2 | 700 | 50 mJ | 1–15 Layers of MoS2 | p-MoS2 2H phase MoS2 Roughness of 0.27 nm |
[71] |
| Si | MoS2 | 4 × 10−4 | RT | 5/10/100/400 mJ/cm2 | ≈100–200 nm | Various compositions of MoSx (x ≤ 2.2) | [85] |
| SiO2 | MoS2 | 3 × 10−5 | 700 | 200 mJ | 1–5 layers | 2H phase MoS2 | [86] |
| W (100)-tip | MoS2+poly(vinl) | 5 × 10−3 | 700 | 2000 mJ/cm2 | ≈20–60 nm | nearly stoichiometric 2H phase MoS2 | [65] |
| n-Si and p-Si | MoS2+poly(vinl) | 5 × 10−3 | 700 | 500 mJ/cm2 | ≈20–60 nm | nearly stoichiometric 2H phase MoS2 | [65] |
| Al, Ag, Ni, Cu | MoS2 | 2.6 × 10−5 | 500 | 50 mJ | ≈5 nm | Epitaxial growth of 2H phase MoS2 | [68] |
| Sapphire Quartz SiO2 HfO2 |
MoS2 +S powder | 1.33 × 10−2 | 700 | 30 mJ | 1 monolayer—2.8 nm | large-area growth of stoichiometric layered 2H phase MoS2 | [87] |
| SiO2/Si | MoS2 | 10−5 | 700 | 200 mJ | few-layer | 2H phase MoS2 | [88] |
| SiO2/Si | MoS2 powder | 5 × 10−4 | 600 | 2200 mJ/cm2 | 13 nm | Epitaxial growth of 2H phase MoS2 | [89] |
| Si | MoS2 | 10−4 | RT | 100 mJ | 129–1900 nm | Stoichiometric films | [90] |
| c-plane sapphire | MoS2 | 10−3 | 800 | 2000–3000 mJ/cm2 | 1–5 layers | Epitaxial growth of 2H phase MoS2 | [91] |
| Quartz glass | Polycrystalline MoS2 powder | 5 × 10−4 | 300 | 8500 mJ/cm2 | 9–10 monolayers | nearly stoichiometric 2H phase MoS2 | [92] |
| Quartz | MoS2 | 8.9 × 10−5 | 600 mJ | ≈5.8 nm | 2H phase MoS2 | [93] | |
| SiO2/Si | MoS2@Ag | 1.33 × 10−7 | 500 | 1000–2000 mJ/cm2 | ≈1.3–12.8 nm | 2H phase MoS2 | [94] |
| fluorophlogopite mica | MoS2 | 10−5 | 700 | 4000 mJ/cm2 | ≈3.3 nm | 2H phase MoS2 | [95] |
| Al2O3 (0001) | MoS2 | 10−3 | 650 | 100 mJ | ≈400 nm | 2H phase MoS2 | [96] |
In addition to the main fabrication methods presented above, other PVD techniques have been used to deposit 2D-MoS2 films. Among these methods, magnetron sputtering has been used to deposit both MoS2 and WS2 films onto polydimethylsiloxane (PDMS) polymer substrates [7][97][98][99][100] with controllable defect densities. The PDMS substrate was chosen to fabricate flexible devices based on 2D-semiconducting materials. Interestingly, very smooth MoS2 surfaces, with a roughness of less than 2 nm, were achieved by casting the polymer on a polished silicon wafer. It has also been shown that it is possible to induce subsequent crystallization of MoS2 by exposing it to a pulsed 532 nm laser [97].
Finally, the use of any of the above-discussed techniques to fabricate 2D-MoS2 films is mostly dictated by the availability of the equipment, expertise, and requirements of targeted application. In a general context, the physical-chemical and optoelectronic properties of the final MoS2 films will be determined to select the appropriate synthesis route. Nevertheless, the level of complexity, throughput, and fabrication costs have to be considered to choose the appropriate synthesis technique particularly when a technology has to be adopted. Table 6 provides a general comparison of the preparation techniques of MoS2 described in this review by listing their main advantages and limitations.
Table 6. Comparison of the advantages and limitations of different preparation techniques of MoS2.
| Techniques | Advantages | Limitations |
|---|---|---|
| Mechanical exfoliation |
|
|
| Chemical exfoliation |
|
|
| Chemical vapor deposition |
|
|
| Atomic layer deposition |
|
|
| Pulsed laser deposition |
|
|
| Sputtering |
|
|
To assess the crystalline quality, microstructure, and optoelectronic properties of the synthesized 2D-MoS2, a variety of characterization techniques have been employed and reported in the literature. These include optical microscopy (OM), scanning electron microscopy (SEM), high-resolution transmission and Scanning transmission electron microscopy (HRTEM and HRSTEM), atomic force microscopy (AFM), energy-dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and photoluminescence (PL). These methods are often used to investigate the overall 2D-MoS2 surface topography and to qualify the nature of the synthesized material and the shapes of its building blocks (i.e., triangle, nanosheets, and nanoplates) (Figure 4). The observations made by imaging methods are also essential to envision a possible growth mechanism of the micro/nanostructures with respect to the used processing parameters. For instance, Figure 4d shows a schematic representation of the nucleation process of some morphologies of 2D-MoS2.
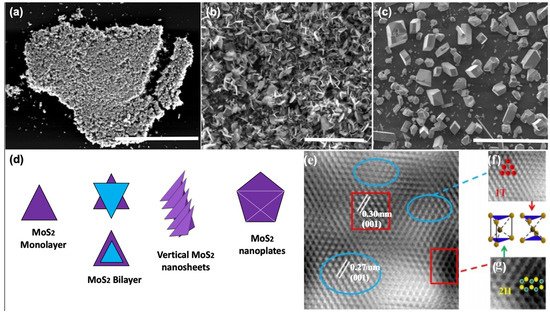
Figure 4. Examples of MoS2 microstructures: (a) planar triangle flakes scale = 40 µm; (b) vertical nanosheets scale = 100 um; (c) vertical nanoplates scale = 100 µm; (d) schematic of the nucleation process of MoS2; (e) HRTEM image of mixed 1T-MoS2 and 2H-MoS2; (f) zoom in of blue circled region of the 1T-MoS2 structure, with the unit cell of the 1T phase; and (g) zoom in of red circled region of the 2H-MoS2 structure, with the unit cell of the 2H phase. (Figure 4e–g adapted from Ref. [101] Copyright 2019, Springer Nature.)
Subsequently, HRTEM investigations could be carried out to precisely characterize the MoS2 crystalline structure and examine locally its lattice parameters and the presence of defects. In particular, the HRTEM image depicted in Figure 4e is of great importance, as it was recorded in cross-region containing the two possible crystal configurations of MoS2. As it can be seen in Figure 4e–g, the identified phase mixture of 1T@2H-MoS2 could coexist simultaneously in the same fabricated MoS2 thin film [101].
AFM and its variant methods constitute key characterization tools for the investigation of 2D crystals, mainly due to the atomically thin nature of this layered class of materials. Both vertical and lateral resolutions are fundamentally required to properly investigate the intrinsic properties of 2D materials. AFM is among the few techniques that allow the characterization of 2D-MoS2 in ambient and controlled environments at the nanometer scale. In addition to measuring the local thickness and surface topography, AFM-based electrical methods provide access to additional interesting properties such as the local variations in surface potential of 2D-MoS2. For instance, the Kelvin probe force microscopy (KPFM) method allows the characterization of the sample’s surface work function variations. The work function is an extreme surface property, which depends on the energy differences between the Fermi and vacuum levels at the surface. This renders the use of KPFM for the characterization of 2D-MoS2 fundamentally important to investigate band alignments in nanostructures and to study the dependencies of local electronic properties on the number of 2D-MoS2 layers. It also provides key insights into the environmental effects on the state of the sample surface both electronically and morphologically. The KPFM technique was used (Figure 5a) to determine the surface potential variations in mono- and multilayer MoS2, under different humidity conditions.
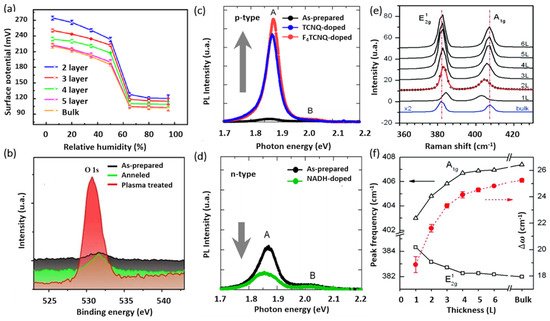
Figure 5. (a) Surface potential captured by KPFM vs. relative humidity RHs with respect of the number of MoS2 layers (reproduced and adapted from Ref. [102], Copyright 2017, IOP Publishing); (b) XPS spectra of Mo 3d and S 2s core levels for different treatment conditions (adapted from Ref. [103] Copyright 2014, American Chemical Society); (c,d) PL spectra of monolayer MoS2 before and after being doped (reproduced from Ref. [104] Copyright 2013, American Chemical Society); and (e,f) Raman spectra for various MoS2 films with respect to the number of MoS2 layers (reproduced from Ref. [105] Copyright 2010, American Chemical Society).
X-ray photoelectron spectroscopy (XPS) is another relevant surface characterization technique that is widely used to achieve the elemental surface composition of MoS2 films as well as their chemical bonding states. Figure 5b shows typical high-resolution XPS spectra of the Mo3d and S2p core levels. The Mo3d region exhibits two characteristic emission peaks at 232.5 (Mo 3d3/2) and 229.4 (Mo 3d5/2) eV. These binding energy values are consistent with electrons of Mo4++ corresponding to MoS2. Likewise, the S 2p3/2 and S 2p1/2 doublet appearing at binding energies of 162.3 and 163.5 eV is typical for S2- in MoS2 structure. Nan et al. [103] used XPS to show the PL enhancement of monolayer MoS2 through defect engineering and oxygen bonding. The chemical adsorption of oxygen created a heavy p-type doping and the conversion of the Trion into Excitons. Moreover, it caused the suppression of the non-radiative recombination of the excitons at the defect sites. Their results were verified by PL measurements at low temperature, as shown in Figure 5c,d.
Unlike bulk MoS2, the ultrathin 2D-MoS2 (i.e., one to few layers) exhibits a strong PL intensity which increases with reducing the number of layers [106], which has been attributed to quantum confinement effects [23][107]. The PL response can be tuned via several mechanisms including doping [104], plasmonic effect, and defects engineering [103]. For instance, Mouri et al. [104] studied the influence of the thickness on the PL response of MoS2 by using mono-, bi-, and trilayer MoS2 and the PL modulation using doping. They demonstrated that p-type doping with high electron affinity seems to enhance the PL intensity, while the n-type doping tends to reduce it, as illustrated in Figure 5c,d.
Moreover, Raman spectroscopy presents a very sensitive, fast, and non-destructive technique to access valuable information on the chemical structure, phase and polymorphs, crystallinity, and chemical bonding states of 2D-MoS2 materials. It allows the monitoring of the two characteristic peaks of MoS2, namely the in-plane and out-of-plane vibration modes E12g and A1g appearing for 514 nm excitation energy at the respective positions of 384.5 and 404.6 cm−1 for 2D-MoS2 monolayer [105] (Figure 5e). More interestingly, the difference between the peak positions of E12g, A1g (Δω) can be used as a robust and effective diagnostic to determine the number of MoS2 layers (up to four layers) or to simply estimate the MoS2 film thickness (Figure 5f). Usually, Δω is less than 20 cm−1 in the presence of a single layer of MoS2, but it increases with increasing MoS2 thickness to reach 25 cm−1 for the bulk MoS2 [105]. In fact, a thorough study on the dependence of the characteristic Raman peak positions, width, and intensity of MoS2 films on their thickness have been investigated [73][105][108]. Furthermore, H. Li et al. [108] reported that the frequency of the characteristic peaks is strongly dependent on the excitation energy due to the resonance effect. They showed a red shift of the E12g mode of about 2.2 cm−1 and blue shift of the A1g mode of about 4.1 cm−1. Thus, to effectively determine the exact MoS2 number of layers using Raman spectroscopy, one has to consider the excitation energy and the thickness limit at which the Raman vibrations frequency is reaching a plateau, indicating that it is less sensitive to MoS2 thickness variation above four layers.
We employed density functional theory (DFT) to determine the optoelectronic properties in particular the bandgap energy of both bulk and monolayer MoS2. Perdew–Burke–Ernzerhof (PBE) approach was applied to describe the electronic states of MoS2 using band structure and the density of states (DOS). DFT calculations were implemented in Quantum Espresso™ code [109][110]. The considered 2H-MoS2 has a hexagonal crystal form with the space group P63/mmc (No. 194). The equivalent positions for this structure employed in the calculations are Mo (1/3, 2/3, and 2/8) and S (1/3, 2/3, and 0.621). The valence electron configuration selected for Mo and S atoms are 4p5 5s1 and 3s2 3p4, respectively. The cutoff wave function and the cutoff charge densities are 70 and 700 Ryd, respectively [110]. The cell parameters and atomic positions were fully relaxed by the process of the total energy minimization. The values of the relaxed lattice constants for bulk MoS2 are a = 3.15 Å and c = 12.3 Å, respectively. The optimized structure was used to perform calculations for band structures and the total density of states for both MoS2 bulk and monolayer. For bulk MoS2 (top left panel of Figure 6a), 9 × 9 ×2 k-points were used to obtain the band structure along the path Γ-K-M-Γ in the Brillouin zone. For MoS2 monolayer (top right panel of Figure 6a), 9 × 9 × 1 k-points were used. A 15 Å vacuum along the z-axis above the monolayer was added to isolate the MoS2 and prevent any interaction between the adjacent layers [111]. The top view of the MoS2 monolayer is shown in the bottom panel of Figure 6a, where sulfur atoms are represented in yellow and molybdenum atoms are shown in purple.
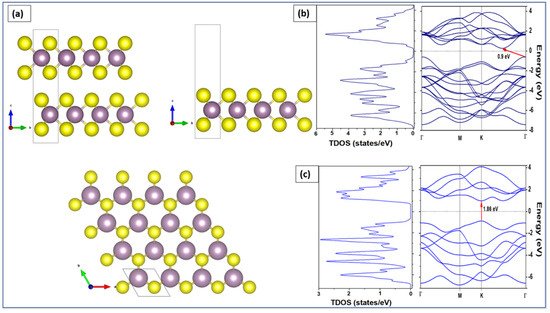
Figure 6. (a) Bulk MoS2 (top-left), monolayer MoS2 (top-right), and top view of MoS2 monolayer (bottom). Total density of states (left) and band structure (right) of the (b) bulk and (c) monolayer.
To obtain the electronic properties, the MoS2 bulk was considered as a set of two hexagonal planes linked together by weak Van Der Waals bonds. The MoS2 monolayer was considered as a single hexagonal plane with covalent bonds between atoms S-Mo-S [112]. The left panel of Figure 6b shows the total DOS calculation results of the bulk MoS2 while the right panel of Figure 6b shows the calculation of its band structure. The energy range is between −8 and 4 eV versus the directions of the highest symmetries in the first Brillouin zone Γ, M, K, and Γ. As observed from the band structure calculations, the MoS2 bulk has an indirect bandgap of 0.9 eV. The minimum of the conduction band is located between K and G and the maximum of valence band at point G. This indirect bandgap obtained for the MoS2 bulk was attributed to the presence of interlayer interactions in the bulk structure [113]. In contrast, Figure 6c shows that the monolayer MoS2 has a direct bandgap of 1.89 eV at the K point. The DOS results are compatible with the results of the band structure. Similar conclusions have been stated in other investigations [111][112].