1000/1000
Hot
Most Recent

The increased demands of small-diameter vascular grafts (SDVGs) globally has forced the scientific society to explore alternative strategies utilizing the tissue engineering approaches. Cardiovascular disease (CVD) comprises one of the most lethal groups of non-communicable disorders worldwide. It has been estimated that in Europe, the healthcare cost for the administration of CVD is more than 169 billion €. Common manifestations involve the narrowing or occlusion of blood vessels. The replacement of damaged vessels with autologous grafts represents one of the applied therapeutic approaches in CVD. However, significant drawbacks are accompanying the above procedure; therefore, the exploration of alternative vessel sources must be performed. Engineered SDVGs can be produced through the utilization of non-degradable/degradable and naturally derived materials. Decellularized vessels represent also an alternative valuable source for the development of SDVGs. In this review, a great number of SDVG engineering approaches will be highlighted. Importantly, the state-of-the-art methodologies, which are currently employed, will be comprehensively presented. A discussion summarizing the key marks and the future perspectives of SDVG engineering will be included in this review. Taking into consideration the increased number of patients with CVD, SDVG engineering may assist significantly in cardiovascular reconstructive surgery and, therefore, the overall improvement of patients’ life.
Small-diameter vascular grafts (SDVGs) with inner lumen diameter (d) less than 6 mm are required in vascular reconstructive surgery. Tissue engineering (TE) represents an emerging research field where the production of vascular grafts utilizing state-of-the-art manufacturing methods has gained great attention from the scientific society [1][2]. In contrast to large (d > 8 mm) and medium (d = 6–8 mm) diameter vascular grafts, which have currently been applied in a wide variety of vascular applications, such as carotid and aorta replacement, the production of SDVGs (d < 6 mm) requires further improvement [1][2][3]. Indeed, synthetic vascular grafts, derived from expanded polytetrafluoroethylene (ePTFE) and Dacron, serving as medium- or large-diameter vessel transplants, have shown interesting results in reconstructive surgery [4]. Long-term results of large diameter vascular grafts (LDVGs), e.g., when applied as aortoiliac substitutes, have exhibited good patency rates (90%) within the first year of implantation [2][5][6]. Additionally, medium-diameter vascular grafts, such as the carotid substitutes, are characterized by patency rates greater than 60% after the 1st year of implantation [2][7]. On the other hand, the proper production and use of small-diameter vascular grafts in reconstructive surgery are still under evaluation.
SDVGs are initially aimed to be used in coronary artery bypass grafting (CABG), issued by manifestations of cardiovascular disease (CVD). Regarding non-communicable diseases, CVD is the most leading cause of death globally [8][9]. CVD is a group of complex disorders, including peripheral arterial disease (PAD), coronary heart disease (CHD), cerebrovascular disease, and rheumatic heart disease [8][10]. It has been estimated that in the European Union (EU), CVD causes more than 3.9 million deaths, which accounts for 45% of all deaths each year [11]. Moreover, 11.3 million new cases of CVD are reported in the EU annually [12][13]. Furthermore, the United States is characterized by an increased percentage of CVD cases and deaths [14][15]. It is estimated that more than 400,000 CABG procedures are performed in the USA annually [14][16]. The CVD occurrence is mostly related to changes in dietary habits, reduced exercise, increased working time, depression, national health care deficiencies and the occurred financial crisis [17][18][19][20]. In terms of economic burden, it has been estimated that in Greece, the mean annual healthcare cost per patient is 5495 €, 4594 €, and 8693 € for CHD, CVD, and PAD, respectively [21]. Therefore, the proper development and clinical utilization of functional SDVGs is of paramount importance.
Nowadays, a great number of treatments can be effectively applied in CVD. These treatments may include the change of dietary–lifestyle habits or the application of pharmaceutical and surgical approaches. In the context of vascular surgery intervention, endovascular approaches such as angioplasty, atherectomy, and stent insertion can be performed. Additionally, vascular graft transplantation may be applied as an alternative option to replace or bypass the injured vessels.
The manufacturing of SDVGs with the TE methodologies has been improved significantly since the first attempts for production and application of synthetic vascular grafts used in bypass surgeries in the late 1980s [22]. Several years later, the first commercially available tissue-engineered vascular grafts (TEVGs) appeared, including Synergraft® (CryoLife, Inc., Kennesaw, GA, USA), Artegraft® (LeMaitre Vascular, Inc., Burlington, MA, USA), Procol® (LeMaitre Vascular, Inc., Burligton, MA, USA), and Cryovein® (CryoLife, Inc., Kennesaw, GA, USA) [23]. The majority of these grafts have received approval from the Food and Drug Administration (FDA) and the European Medicinal Agency (EMA) for human applications.
The proper design of the vascular grafts ensures successful cell seeding at the pre- and post-implantation stage. Cellular populations may positively influence the vessel graft functionality [24]. The most applied cellular populations are the endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) [25]. ECs are located in the internal layer of the vascular wall, known as tunica intima, forming the endothelium [26]. The endothelium has unique anti-thrombogenic properties, avoiding the platelet aggregations and clots formation [27]. VSMCs are responsible for vasoconstriction and vasodilation, located in the media layer of the vessel wall, which is known as tunica media [28]. Dependent on microenvironment stimuli, the ECs can elevate the levels of endothelial nitric oxide synthetase (eNOS), leading to NO production, which downstream induces the VSMCs-dependent vasodilation [27]. Importantly, VSMCs also support the vascular remodeling and regeneration with the production of extracellular matrix (ECM) proteins such as collagen and elastin [28]. Besides, the beneficial effects of the cellular populations may occur to the vascular graft, their successfully seeding and proliferation may require long-term cultivation periods. Additionally, the isolation and expansion of specialized cellular populations from patients with CVD is a demanding challenge [29]. To date, there is a tendency for developing readily available acellular vascular scaffolds with improved anti-thrombogenic properties [30][31][32][33]. Indeed, these pioneering studies are focusing on the fabrication of a negatively charged synthetic surface in order to avoid red blood cells and platelet aggregation. In this way, the SDVGs must satisfy certain design criteria to be clinically available [34]. Specifically, SDVGs must have similar biomechanical properties (burst pressure, high-stress deformation, and suture strength) with the substituted vessels to avoid aneurysm and neointima development [35]. In addition, regardless of the vascular graft material, engineered vessels must be non-cytotoxic and support cell growth [34]. Engineered SDVGs must be characterized by specific ultrastructure, ensuring the cell seeding, proliferation, and differentiation [2]. Finally, the engineered SDVGs must not be immunogenic, and also must be characterized by in vivo remodeling and regeneration properties [2].
Nowadays, a wide variety of manufacturing techniques for SDVGs such as the use of synthetic polymers, decellularized natural matrices, bioprinting, and 4D printing have been developed, although the majority of them require further evaluation and optimization.
The TESA approach was developed by the pioneer L’Heureux and aimed at the production of vascular grafts utilizing cell sheets [36]. To achieve this outcome, no supporting vascular scaffolds are required.
The basic concept has relied on the use of cell sheets containing fibroblasts, mesenchymal stromal cells (MSCs), ECs, and VSMCs, which were shaped around a mandrel to produce a tubular formation (Figure 3). Further maturation in the pulsatile bioreactor is required in order to vascular grafts to achieve the prerequisite burst pressure and overall mechanical properties [36][37]. The initial work of L’ Heureux et al. [38] involved the cultivation of SMCs and fibroblasts in a standard culture medium contained sodium ascorbate. One month later, the produced sheets were shaped with the use of a tubular mandrel. The same technique was applied for the production of the different layers of the vascular graft. The results of this study were impressive. Specifically, histological analysis revealed the proper localization of the cellular populations, while the burst pressure of the produced vascular conduit was more than 2500 mmHg [38]. Moreover, these vessels were implanted as femoral artery interposition grafts in canine animal models, withstood the blood flow, and met the fundamental requirements of a vascular graft. More experiments also were performed utilizing human cells for the development of vascular grafts and their testing in different animal models. The above results led to the performance of the first clinical trial with TESA-produced TEVGs [38].
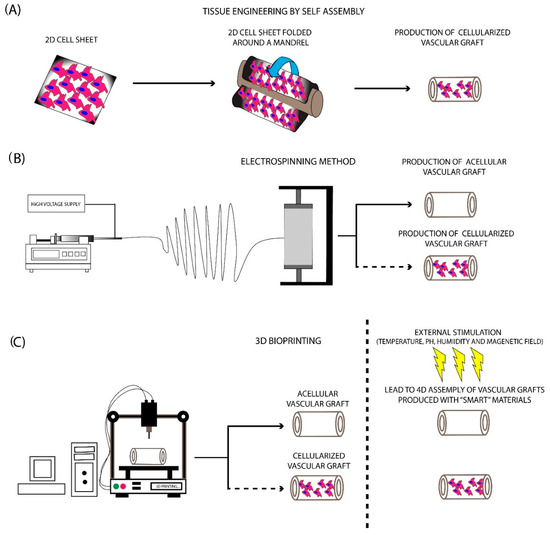
Figure 3. Fabrication methods for the production of SDVGs. (A) Production of SDVGs with the originally proposed method of L’Heureux et al. In this method, the production of SDVGs was relied on the self-assembly of cell sheets using a tubular mandrel. (B) Production of SDVGs with the electrospinning method. This methodology can produce complicated extracellular matrices (ECMs). In addition, combination with cellular populations can lead to the development of cellularized structures. (C) Production of SDVGs with the bioprinting method. Bioprinting offers the potential for the production of either acellular or cellularized complicated structures. Moreover, when used “smart” materials in the production process, the final product can assembly on the desired structure upon external stimulation (e.g., temperature, pH, humidity, and magnetic field).
The electrospinning method comprises the first attempt to mimic the complex structure of natural ECM. This method was introduced in 1930, providing an economical solution for scaffold fabrication [39]. Nowadays, its use has been expanded, thus scaffolds for bone and cartilage regeneration can be manufactured efficiently. Electrospinning has relied on the production of nano- and microfibers derived from a viscoelastic solution, where a high electrostatic force is applied. More specifically, the material that will be electrospun is pumped at a slow rate, ending in a high voltage electrical field [23][40][34,232]. This, in turn, leads to charging the polymer material during its exit from the syringe, which results in the production of the Taylor cone. A narrow jet of liquid is generated from the Taylor cone, which is further collected to a specific set up, known as the collector (Figure 3). Finally, the production of a scaffold, characterized by adequate ECM structure and fine-tuning mechanical properties, is produced [2][23]. The formation of the produced fibers is affected by various parameters, which are specific for the material, used each time, including molecular weight, surface tension, density, and viscosity [41]. Except for those, other parameters that can affect the fiber composition mostly include the applied electrostatic field, temperature, humidity, and flow rate of the polymers [41].
The polymer materials used in the electrospinning approach could be either degradable or natural derived materials [2][23]. However, important differences between the different materials exist. In the past, degradable materials such as PLA, PGA, PCL, PU/silk fibroin have been used for the production of scaffolds and specifically tubular conduits utilizing the electrospinning approach [2][23]. Vascular grafts have also been fabricated with the electrospinning method. Importantly, the proper combination of PLGA with collagen type I and elastin can improve the mechanical properties of the produced scaffolds, and their use is preferred for the production of electrospun blood vessels [42]. Moreover, it has been shown that the addition of naturally derived materials, such as collagen, gelatin, and fibronectin, may provide more RGD-binding sites, thus improving the cellular functions, like adhesion, growth, and differentiation [43].
In the context of electrospun tubular scaffold application, both acellular and cellularized conduits have been evaluated. Wise et al. [44]developed a tubular scaffold consisted of tropoelastin and PCL with the electrospinning method. The produced scaffold was characterized by similar biomechanical properties as the internal mammary artery (IMA). Further investigation involved the implantation of the acellular conduit in animal models [44]. Furthermore, the biomechanical analysis was performed in electrospun vascular grafts pre- and post-implantation. Specifically, acellular electrospun vascular conduits were implanted as carotid artery interposition grafts in rats for a total period of 1 month. Histological analysis in the explants showed the successful recellularization of the vascular grafts with ECs. Moreover, the explanted electrospun vascular grafts were able to preserve the initial vessel morphology and characterized by similar biomechanical properties as the pre-implanted grafts. In this study, the successful accumulation of tropoelastin in PCL scaffolds was shown for the first time, resulting in the production of vascular grafts, which were characterized by impaired platelet adhesion and increased endothelialization [44]. Additionally, Soletti et al. [45] provided substantial evidence regarding the proper development and production of anti-thrombogenic vascular conduits. Soletti et al. [45] showed that the acellular poly(etherurethane urea) (PEUU) grafts coated with the non-thrombogenic 2-methacryloyloxyethyl phosphorylcholine copolymer showed better patency and mechanical properties compared to uncoated PEUU vascular grafts [45]. Unlike Wise et al. [44] and Soletti et al. [45], Min Ju et al. [46] managed to develop electrospun bilayer tubular scaffolds consisted of PCL and collagen type I. Then, ECs and SMCs obtained from female Dorper Cross Sheep were seeded onto the tubular scaffolds, followed by maturation in the pulsatile flow bioreactor. The seeded vascular grafts were implanted as carotid artery substitutes in the sheep model and remained for 6 months. The electrospun vascular grafts were remained patent and the histological analysis revealed the production of collagen, elastin, and glycosaminoglycans within 6 months of implantation [46]. This study provided valuable data regarding the production and application of the electrospun vascular grafts. Moreover, Du et al. [47] used the electrospinning method to fabricate a 3D vascular microenvironment. In this approach, immobilization of VEGF onto the electrospun tubular scaffold consisted of gradient chitosan and PCL nanofibers was performed. The controlled release of VEGF potentially can enhance the adhesion of ECs and SMCs and further promote their rapid proliferation. In this way, engineered SDVGs with improved anti-thrombogenic properties could be developed, leading to the avoidance of lumen occlusion and thrombus formation, a series of common manifestations which are presented several days after the vessel implantation [47]. Taking into consideration the above data, it was clearly shown that electrospinning could be applied for the efficient production of engineered SDVGs. The produced electrospun SDVGs could be successful in vitro seeded with cellular populations and maintain further their graft patency, mechanical properties, and vessel integrity over a long time period.
In the last decade, 3D printing technology has gained significant attention and has been utilized with great success in a wide range of applications [48]. Using this technology, complex structures and materials can be produced efficiently, thus can be further used by the scientific society. The evolution of printing technology is 3D bioprinting, which has currently been applied in various tissue engineering approaches [48]. 3D bioprinting can produce complex structures, utilizing non-degradable/degradable and naturally derived polymers [49]. The significant potential of this methodology is the production of ready to use transplantable scaffolds and tissues. Currently, the 3D bioprinting approaches such as inkjet, extrusion, and laser-assisted bioprinting are mostly used for the production of the majority of the scaffolds [49]. A great series of materials are compatible with the bioprinter applications, although the polymer materials are mostly preferred in comparison with the naturally derived materials [49][50]. The bioprinter materials can be distinguished into three categories: (a) fibrous materials, (b) powder materials, and (c) bioinks. The use of the starting material is dependent on the characteristics of the produced scaffold [49][50].
3D bioprinting approaches and the proper combination of the aforementioned materials have been successfully applied in the production of LDVGs and SDVGs. [49][51][52][53]. In this direction, Freeman et al. [54] presented for the first time a new approach for the development of SDVGs using a custom-made 3D bioprinter. In this study, gelatin and fibrinogen were properly combined, producing a bioink with good rheological and printability properties. The produced vascular graft provided a favorable ECM for cell attachment. However, comprehensive in vitro and in vivo evaluation is further needed to be performed [54]. Jia et al. [55], in their study, used a multilayer coaxial nozzle device to produce vascular grafts. Moreover, human umbilical vein endothelial cells (HUVECs) and MSCs were expanded and encapsulated in a gelatin methacryloyl (GelMA), sodium alginate, and 4-arm poly(ethylene glycol)-tetra-acrylate (PEGTA) based bioink. Using the current bioprinter set-up in combination with the developed bioink resulted in the printing of highly organized vascular structures. No sign of cytotoxicity was reported, and after a time period of 21 days, the cells filled the entire printed vascular grafts. To evaluate better the cell behavior into the vascular wall, immunofluorescence was performed, showing the positive expression of α-SMA and CD31, in MSCs and HUVECs, respectively [55].
Next-generation bioprinting demands the use of materials capable of self-transform into a prerequisite shape in order to exert their key functional properties. This state-of-the-art approach is known as 4D bioprinting and has gained increased attention in the last decade by the entire scientific community [56]. 4D bioprinting uses the same materials as conventional 3D printing approaches [49]. The major difference between 3D and 4D bioprinting is that the latter exerts a “smart” behavior of the produced scaffolds [57]. 4D produced scaffolds are superior to the conventionally bioprinted scaffolds. The “smart” behavior corresponds to “materials that can change their physical or chemical properties in a control and functional manner upon exposure to an external stimulus” as has been referred to by Tamay et al. [49]. In this way, 4D printed materials upon exposure to external stimuli such as pH, heat, magnetic field, light, and humidity can adopt effectively different shapes, exhibiting different properties [58]. The above-mentioned factors are playing important role in scaffold’s shape-changing properties. There exists a great variety of materials that achieve shape-transformation in response to temperature stimuli. Thermoresponsive materials are the most commonly used in 4D bioprinting applications [59]. These materials can be distinguished into (a) shape memory polymers (SMP) and (b) responsive polymer solutions (RPS). The first category involves polymers consisting of two distinct components, the elastic segment which is characterized by high glass transition temperature (Tgh), and the switching segment, characterized by intermediate glass transition temperature (Tgi). When the applied temperature is above the Tgh, the produced scaffold adopts its permanent shape. On the other hand, when the temperature is between Tgi and Tgh, the switching segment becomes soft, while the elastic segment resists any shape-changing [60]. Additionally, if the material is cooled below the Tgh, then the elastic segment cannot return to its initial shape and the produced scaffolds acquire its final definitive form. SMPs include mostly the poly(ε-caprolactone) dimethacrylate (PCLDMA), polycaprolactone triol (Ptriol), and poly(ether urethane) (PEU). These materials have been used mostly in applications such as bone and cartilage engineering. RPS is characterized by a critical solution temperature, where if the applied temperature is above the aforementioned temperature (critical solution temperature), the polymer chains are contracting and the overall solution is adopting a solid form [56].
Both hydrophobic and hydrophilic interactions are existing between the polymer chains. In addition, a change in temperature may affect the behavior and the interaction of the above polymer chains. This, in turn, leads to shrinkage or expansion, which is a characteristic of each polymer material. In this direction, a material with a critical solution temperature above 25°C, when implanted to a mammalian organism, would expand, acquiring its definitive form. Poly(N-isopropylacrylamide), poly(ethylene glycol), collagen, gelatin, and methylcellulose are some of the most used RPS [58].
Besides the temperature stimuli, materials that can respond to pH changes also can be widely applied in the clinical setting [56]. The initial structure of these materials is consisting of acidic or basic groups, which are the main players in proton exchange upon pH changes [61]. In this way, polymers consisting of acidic groups, when exposed to pH > 7, act as anionic compounds, while polymers with a basic group, exposed to pH < 7, act as cationic compounds. Therefore, these materials upon pH stimuli can acquire different structural and functional properties, including change in solubility, degradability, swelling, etc. [57]. Like thermoresponsive polymers, pH-responsive polymers also can be utilized in a wide range of applications. Indeed, different human body compartments are characterized by different pH in order to serve properly their initial function, including the gastrointestinal tract, stomach, small intestine, and different regions of the vascular system including the kidney vascular network. Additionally, many solid tumors induce pH changes upon their growth. In this way, pH-responsive polymers can act as DDS, delivering tumor-specific therapy, such as signaling and cell proliferation inhibitors or monoclonal antibodies [62]. Examples of the most commonly used materials in this category are poly(acrylic acid), poly(aspartic acid), poly(L-glutamic acid), and poly(histidine), which can be combined effectively with naturally derived materials such as collagen, gelatin, and chitosan [61].
Besides the aforementioned, other categories of responsive materials have also been manufactured. These categories mostly include the photoresponsive, magneto-responsive, and humidity-responsive materials. However, their potential use is limited and, therefore, further evaluation of their properties is clearly needed. Briefly, photoresponsive materials can change their structural and functional properties, including wettability, solubility, degradability upon photo-stimulation [49]. Considering this, polymer materials with photosensitive groups can be manufactured, where the produced scaffolds can swell or shrink when specific photo-stimulation is applied. The combination of magnetic particles with polymer materials results in the development of magneto-responsive materials. The most commonly used magnetic particles are iron (Fe), nickel (Ni), cobalt (Co), and their oxides [58] Currently, magneto-responsive materials have been used as targeted therapeutic vehicles, carrying anti-tumor drugs. On the other hand, significant adverse reactions may be induced by their use in living organisms [63]. It has been shown that magnetic particles with a size less than 50 nm are transportable through the biological matrix, which can further cause inflammation and cell death due to high reactive oxygen species (ROS) production, DNA damage, and cytochrome C release. In this category, materials such as Fe3O4/PCL, Fe3O4/poly (ethylene glycol diacrylate), PCL/iron doped hydroxyapatite (PCL/FeHA) are currently evaluated for their potent use in living systems [63]. Lastly, humidity responsive materials also have been proposed for their use in 4D bioprinting and the production of tissue-engineered scaffolds. Interestingly the change in humidity could result in shape-change modification, which can act as a driving force for movement. These materials have not received great attention from the scientific community due to their limited use. Humidity responsive materials include poly(ethylene glycol) diacrylate, cellulose, polyurethane, and their combinations [64].
The 4D bioprinting comprises an important evolution in the fabrication of tissue-engineered scaffolds. Vascular grafts can be developed with this next-generation approach. In this way, we can imagine the development of a 4D bioprinted vascular graft (with a large or small diameter), which can acquire its specific shape inside the living organism upon temperature stimulation. Moreover, changes in pH of the vascular network may stimulate the implanted vascular graft in a way either to acquire a different shape or to substantially release key therapeutic agents in order to reduce or even to reverse the occurred situation. In the future, “smart” telebiometrics vascular grafts will be plausible to be employed, which can detect the changes of human body conditions, like temperature, pH, osmolarity, and will be able to notify or even to reverse a health issue. Currently, the utilization of “smart” materials and the manufacturing of those scaffolds is under the developmental stage [56]. Therefore, no significant number of publications is currently existing, with the only exceptions of reviews and opinion articles in this field. In this way, the development of “smart” materials that can be used in vascular engineering is quite important, but further exploration of this research field is needed.