1000/1000
Hot
Most Recent

Nanotechnology and nanoparticles are found to be very effective because of their unique chemical and physical properties and high surface area, but their high cost is one of the major hurdles to its wider application. So, the synthesis of nanomaterials, especially 2D nanomaterials from industrial, agricultural, and other biological activities, could provide a cost-effective technique. The nanomaterials synthesized from such waste not only minimize pollution, but also provide an eco-friendly approach towards the utilization of the waste.
Nanotechnology is providing new solutions and opportunities to ensure sustainable energy and environments for the future. Nanotechnology deals with the design and development of the materials at the nanoscale (1–100 nm) or one dimension in the nanoscale [1][2]. The word nano was derived from the Greek word meaning “dwarf” [3] and denoted as nm. By using such measurement, the size of viruses are about 100 nm (30–100) nm [4] and that of a human hair is 1000 nm in diameter. Nanotechnology and nanoscience allow researchers to manipulate the properties of materials at the atomic level [5]. Nanomaterials are typically those materials having at least anyone dimension at the nanoscale (<100 nm). Nanomaterials can be produced in a variety of methods like chemical, physical and biological with different classes such as carbon-based nanomaterials: carbon-based nanomaterials [6][7], nanocomposites [8], metals, alloys, nanopolymers [9][10], nanoglassses [11], and nanoceramics [9][12]. Nanomaterials can be either synthesized in the laboratory or could be derived from the natural resources [13]. The nanomaterial synthesized from the commercial precursor materials makes the product as well as process expensive. Moreover, the source of nanomaterial is also depleting, so there is a need to rely on the natural and alternative sources of nanomaterial [14]. The natural nanomaterial [15] act as a potential candidate for the development of nanomaterials. The nanomaterial derived from such processes are cost-effective [16], biocompatible [17] and environmentally friendly [13]. The waste materials that are commonly used for nanomaterial synthesis include industrial waste like fly ash [7][18], red mud, agricultural waste [19][20] (rice husk and straw, wheat husk and straw, coconut shell), and plastic waste [21][22]. Most of these waste materials mainly act as a pollutant to the environment, which are produced in tonnes annually around the globe. The utilization of such products for the synthesis of carbon nanomaterials reduces the pollution from the environment and simultaneously provides an environment-friendly and economical approach. Moreover, nanomaterials derived from such waste products will have a greater impact in the industries when these are surface functionalized or transformed into some isomeric forms. The surface functionalization is mainly done for a specific function, by modifying functional groups, etc. These nanostructured materials based on their purities can find applications in electronics [23], wastewater treatment [24], medicine [25], and catalysis [25].
Nanostructured materials (NSMs) have gained a huge consideration in fundamental science and technological applications due to their multifunctionality and unique chemical, physical, electronic and magnetic properties at the nanoscale [26]. Every day, novel nanomaterials are synthesized, so classification is of the utmost necessity. Figure 1 presents the broad classification of nanomaterials.
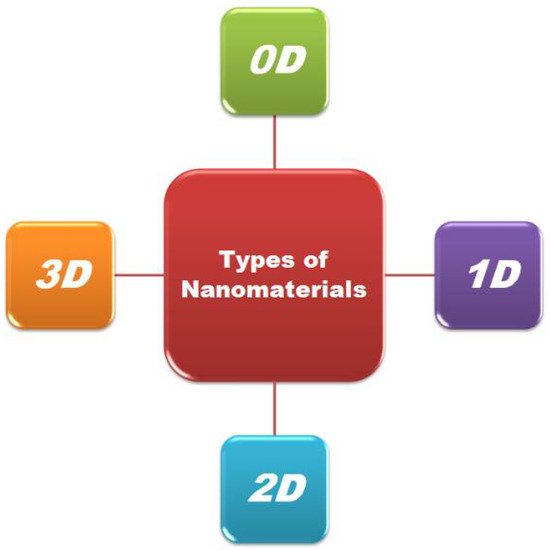
Figure 1. Classification of nanomaterials.
The density of the state varies considerably for different nanomaterials which are based on the degree of freedom/confinement [27]. Based on the nanostructural elements and their physical and chemical properties, the nanomaterials have been classified into four classes, i.e., 0D, 1D, 2D, and 3D, by Pokropivny.
In 0D material (quantum dot) [QD], there is confinement of electrons in all three directions [28]. Zero dimensional nanomaterial has gained huge attention in the field of research and in material-based industries [29]. Such material finds applications in the light-emitting diodes (LEDs) [30], solar cells [31], single-electron transistors [32], and lasers. The common example of zero-dimensional nanomaterial are spheres (including hollow spheres) and nanoclusters [33], quantum dots that includes core-shell QDs also [34], heterogeneous particles arrays, onions [35], and nanolenses. Carbon-based materials such as Fullerene like (FL) structures are having extraordinary mechanical properties and they are being used in multiple applications like biomedicine and microelectronics [36].
One dimensional nanomaterial is those materials which are confined in two dimension but free in one dimension [37]. Some of the common examples of 1D nanomaterial are wires, nanowires [38], nanotubes, nanofibres [39], nanobelts [40], nanoribbons [40], nanorods [41], and hierarchical nanostructures. For the last decade, such nanomaterials have garnered huge attention because of their remarkable properties and such wider applicability in terms of research and development. Such materials have a wider impact in nanoelectronics [42], nanodevices, and nanosystems [43], nanocomposite materials [44], and alternative resources of energy. The 1D nanomaterials are the most preferred material for exploring the properties at the nanoscale. It is also used for the investigation of size and dimensionality dependence of functional properties [45].
2D nanomaterials have only one dimension in the nano range while the other two dimensions are out of the nano range [46]. They are said to be the thinnest materials, which possess the highest surface area, and are increasingly gaining global interest from fundamentals of physical sciences, chemistry, to materials engineering. Graphene was the first 2D nanomaterial to trigger the research on 2D nanomaterial. Other than graphene, researches also focus on other 2D nanomaterials, such as boron nitride, transition metal dichalcogenide, mono-elemental 2D semiconductors, i.e., silicene, germanene, stanene, and phosphorene, and 2D oxide/hydroxide materials. In recent years, not only the synthesis, but also the applications of 2D NSMs have drastically drawn attention in materials research because of their several interesting properties at the nanoscale. In comparison with bulk materials, two-dimensional (2D) nanomaterials own rare physiochemical assets develops due to their high aspect ratio (SVR) [47], distinctive surface chemical properties, and quantum confinement effect [48]. The 2D NSMs finds applications in sensing materials, photocatalysis, nanocontainers and nanoreactors [34]. Most preferably, the metallic based 2D NSMs have been exploited widely in sensing, catalysis, photothermal therapy, surface-enhanced Raman scattering (SERS), bioimaging, and solar cells [49], due to their phenomenal properties. The common examples of 2D nanomaterials are nanoprisms [50], nanoplates [51], nanosheets [52][53], nanowalls [53], and nanodisks [54], which are shown in Figure 2.
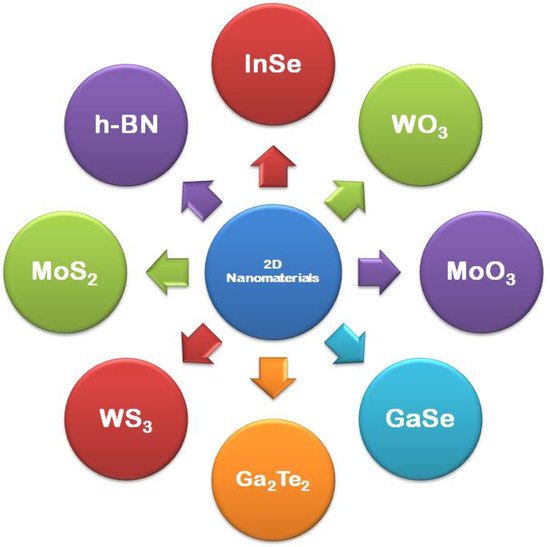
Figure 2. Examples of 2D materials.
The 3D NSMs three dimensional nanomaterials are those materials that have their free dimensions in all three directions and there is no confinement and limitations [34]. The common examples of three 3D nanomaterials are powders [55], multilayer [56], fibrous and polycrystalline material [57]. The 3D nanomaterials exhibit a large specific surface area [58], because of which such nanostructures provide adequate surface absorption sites for the molecules in a small area. The 3D NSMs are extensively used for catalysis in nanomaterials finds applications in the field of catalysis [59], magnetism and for the development of electrode material for batteries [60]. Additionally, the porosity in the three dimensions supports the easy transport of the molecules. Examples of 3D NMs include nanoballs (dendritic structures) [61], nanocoils [62], nanocones [34], nanopillers [63], and nanoflowers [63].
The nanomaterials could be developed by all three means i.e., chemical, physical and biological methods which is shown in Figure 3. Among them, the physical approaches include sputtering [64], laser ablation [65], pyrolysis [66], lithography [67], and hot and cold plasma [67]. Meanwhile, the chemical methods that are used most frequently are lyotropic liquid crystal templates [68], electrochemical deposition [69], electroless deposition [70], hydrothermal [71] and solvothermal techniques [72], sol gel technique [73][74], laser chemical vapor deposition technique [75], laser pyrolysis [76], and chemical vapor deposition [77]. The nanomaterials could also be synthesized by biological approaches like microbial [78] and plant derived materials [79]. The microbial synthesis of nanomaterials [80] employs the utilization of microorganisms like algae [81], fungi [53], and bacteria [82]. The main drawback is that when there is a utilization of commercial precursor for the synthesis of nanomaterials by any of the above-mentioned approaches, the process, as well as the product, become expensive. So, in order to obtain a cost-effective material, the precursor should be lower in terms of cost. One such material is industrial waste [83], biological waste, or agricultural waste [19].
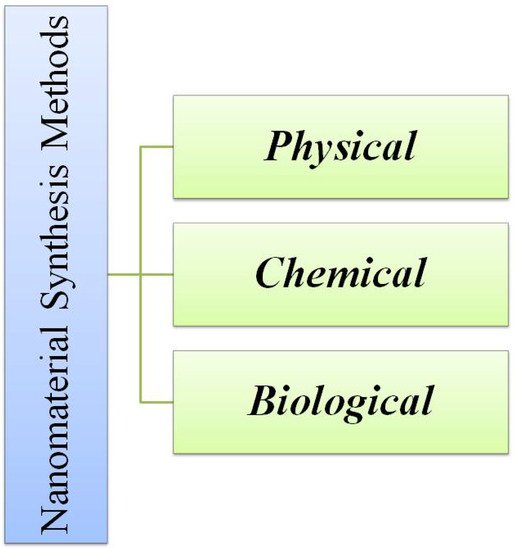
Figure 3. Different methods of synthesis of nanomaterials.
The 2D NSMs could be synthesized by various physical methods [84] such as evaporation [85], lithography [86], sputtering, phase condensation, hot and cold plasma spray pyrolysis [87], inert gas phase condensation [88], pulsed laser ablation method [89], and sonochemical reduction [90]. These methods (physical) are generally used for the synthesis of nanowalls [53], nanoprisms [91], nanosheets [92], nanoplates [93], and nanodisks [34]. The nanomaterials synthesized by the physical method are homogenous in nature and ordered. Dai et al., 2002 developed the SnO nanodisks [64] alumina plates using the thermal evaporation method under optimized environmental conditions [94]. Here, firstly, SnO or SnO2 powders were kept in an alumina boat, which was in turn placed in a quartz tube reactor (evaporation source), where alumina acted as a substrate which was placed one by one downstream. The physical techniques provide an environment-friendly approach for the development of 0D, 1D, 2D, and 3D nanomaterials, which are shown below in Figure 4.
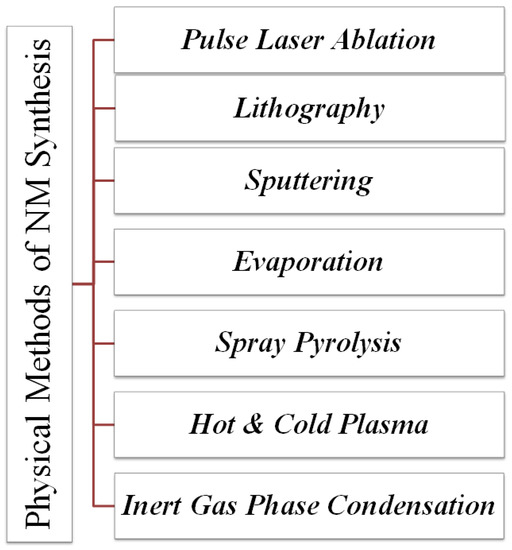
Figure 4. Physical methods for the synthesis of 2D nanomaterial.
Chemical methods have contributed to the fabrication of materials at the nanoscale [95]. The Chemical methods have several advantageous properties over physical methods as the previous one involves mixing of chemical at the molecular level which ensures good chemical homogeneity [74][96]. Chemical reduction methods are reported to have numerous drawbacks for instance utilization toxic reagents and solvents, generation of unwanted by-products due to which there are several extra steps is needed for removal of impurities, time-consuming [97]. The most common chemical methods are electroless deposition [98], lyotropic liquid crystal templates [34], hydrothermal and solvothermal method, sol-gel technique, electrochemical deposition, chemical vapor deposition (CVD), laser pyrolysis, and laser chemical vapor deposition techniques (LCVD), which are utilized frequently for the production of different NSMs. The above-mentioned techniques are shown in Figure 5.
Figure 5. Chemical methods of synthesis of nanomaterials.
Chen et al., in 2018, reported the synthesis of two-dimensional metallic nanomaterials from various routes [99]. Yang et al., in 2019, reported the synthesis, engineering, and applications of fly ash from various routes like physical and chemical but the emphasis was given mainly on the precursor mediated synthesis, not on the waste-based materials [100]. In 2015, Paul et al. reported the thin film deposition of Feo on the Pt(111) by the ferrocene adsorption and oxidation method [101]. Zhang et al. reported the synthesis of multifunctional flexible 2-dimensional carbon nanostructured N-nets reported their importance in electronics, energy, and the environment [102].
Among all the metallic nanoparticles silver nanoparticles has gained used consideration due to their exceptional properties and applications. Silver nanoparticles of different shapes and sizes have an important role in medicine, the biomedical field and drug delivery [103]. Till now silver NPs of various shapes and sizes has been reported by numerous investigators. Nanoprisms are one of the examples of 2D nanomaterial, which had gained huge attention in the biomedical field [103]. Silver nanoprisms were synthesized silver salts by chemical reduction and photochemical method where the earlier method is more preferred than the later one due to their more controlled growth of nanoprisms which finds application in the industries [104]. Monodispersed hematite (a-Fe2O3) nanodiscs of size (50 ± 10 nm in diameter and thickness of 6.5 nm) synthesized under mild conditions through a facile hydrothermal method, i.e., hydrolysis of ferric chloride [105]. The reported method was quite unique as there was no use of surfactants, no toxic or hazardous chemical precursors, and no high temperature decomposition of iron precursors in non-polar solvents. The synthesized hematite nanodiscs were further characterized by atomic force microscopy (AFM), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer–Emmett–Teller (BET), and superconducting quantum interface device (SQUID). The synthesis of Ta3N5 nanoplates was reported by Jie Fu and Sara E. Skrabalak, 2016, for the photocatalytic application [106]. A simple technique developed for the production of hexagonal-shaped Ag nanoplates whose diameter was in the range of 15–20 nm with a smooth nanobulk of 120 nm [107]. The silver nanoplates were prepared by a kinetically controlled solution growth method under the following conditions: polyvinyl pyrrolidone (PVP) as a capping agent, dextrose as a reducing agent, and urea as a habit modifier at 50 °C and the crystalline structure of silver nanoplates analyzed by the XRD and TEM.
Xin He et al., 2009 synthesized triangular/hexagonal silver nanoplates, nanobelts and chain-like nanoplate assemblies by utilizing N,N-dimethylformamide (DMF) along with PVP [108]. The results revealed that due to the strong interaction between Ag+ and PVP, there was the formation of individual nanoplates and external features of nanoplates were controlled by the ratio of AgNO3 and PVP. Sial et al., in 2018, synthesized multimetallic nanosheets which were utilized for the manufacturing of fuel cells [109]. Zheng et al., 2011 synthesized Palladium NSs by using CO as a reducing agent [110]. Yan-song Zhou et al., in 2016, reported an ultra-facile and generalized approach for the synthesis of metal oxide nanosheets (TiO2, Co3O4, Fe2O3, ZnO) with a larger surface and applied them for energy applications [111]. Jianxing Liu et al. reported the synthesis of hematite nanosheets by using a large-sized particles of iron red and found that the shape of hematite have important effect on the magnetic and optical properties [112]. All the above-mentioned chemical processes revealed a simple, reliable, and useful approach towards the synthesis of 2D NSMs. The shape, size, and composition of the 2D NSMs can be varied by precursor solutions, conditions of deposition and substrate materials [84].
Besides all the above-mentioned techniques for the synthesis of nanomaterials, there are a few less applied chemical mediated approaches. One such technique is electrochemical synthesis mainly by anodization and cathodization. Though both the techniques are commonly used in an electrochemical based industry but rarely known for the synthesis of nanomaterials. Several investigators have reported the synthesis of 1D, 2D, and 3D nanomaterials by using electrochemical methods. Dai et al., in 2019, reported the synthesis of a 1D nanomaterial by anodization method, and also highlighted their importance for manufacturing energy storage devices. Anodization is an electrochemical oxidation technique for depositing metal, metal oxides or semiconductors on a surface in order to increase the thickness of the metal. Nowadays, porous materials are also synthesized for enhanced applications in the field of energy and wastewater treatment. By using this technique, mainly nanotubes are synthesized. The anodization mechanism depends on the various physical parameters like pH, time, potential, electrolyte temperature and water content. All these factors govern the morphology, porosity, wall thickness, and length of the synthesized nanomaterials. Till now, by applying such a technique, the following metal and non-metal oxides have been synthesized: ZnO, ZrO2, α-Fe2O3, WO3, Ta2O5, Nb2O5, HfO2, CuO/Cu2O, and NiO [113]. Kawde et al. reported the synthesis of AuNPs modified-graphite pencil electrode by cathodization. Further, it was used for the non-enzymatic sensitive voltametric detection of glucose [114]. Numerous investigators also reported the electrochemical based synthesis of either 1D, 2D, or 3D nanomaterials [115][116][117].
The biological synthesis of nanomaterials involves the synthesis from plants and their parts, microbes, e.g., algae, fungi, and bacteria. In comparison to the chemical and physical methods, biological methods are eco-friendly and there is a minimum utilization of hazardous chemicals. Besides this, the nanomaterials synthesized by biological methods are biocompatible. There are several reports wherein the nanomaterials have been synthesized by biological methods [116][118].
Carbon is not only the most abundant element on Earth’s crust, but it also acquires exceptional properties because of its hybrid orbitals. The allotropes of carbon are mainly due to the hybridization of bonds formed after the combination of atomic orbitals (s and p) into new hybrid orbitals as sp, sp2, and sp3. The different allotropes of carbon are buckyballs (0D), CNTs (1D), graphene sheets (2D), and diamond (3D) [119]. Due to the allotropy, carbon forms a separate class of 2D nanomaterials that includes graphene, GO, CNTs, buckyballs and its derivatives which are shown in Figure 6 and the properties of graphene oxide (GO) is shown in Figure 7. All these nanocarbons find applications in electronics, environmental cleanup, drug delivery, agriculture, research and catalysis [120]. The wider applications of nanocarbons are also due to the presence of their wide range of structural and textural properties. Above all, nanocarbons, CNTs, and graphene are the most widely used nanomaterials in the field of nanotechnology [120].
Figure 6. Different types of nanocarbons.
Figure 7. Properties of graphene.
Fly ash is an industrial waste, which is produced during the production of electricity from the pulverized coal in the thermal power plants (TPPs) (TPPs) [18]. Every year, a million tonnes (MTs) of coal fly ash (CFA) are produced throughout the whole world, out of which about 50–60% are utilized while the rest are dumped in the fly ash ponds in the near vicinity of TPPs. These fly ashes act as a rich source of carbon materials like fullerene, CNTs, graphene etc. Such carbon nanomaterials are formed from the organic content of the coal and incomplete combustion of coal, soots, and charcoal combustion end products [12][121]. The unburned carbon could be either directly reused in the TPPs furnace as a fuel or it could be processed further for the synthesis of fullerenes. Other than coal combustion, industrial activities such as mining and metallurgical operations also contribute to fly ash generation. Compositionally, fly ash comprises diverse minerals and carbon materials either in single or combined form. The toxicity risk of fly ash has recently been reported in relation to a deteriorating environmental quality in many developed and developing nations across the globe. In these circumstances, a potential utilization of these materials towards preparation of nanomaterials like CNTs [122], fullerenes [64] and several others could be a significant breakthrough remedy to improve the pollution and toxicity extents and contents of environment. There are numerous researches where fly ash have been used either for the recovery of fullerenes or synthesis of fullerenes. Alam et al. reported the recovery and synthesis of fullerenes from CFA [7]. The nature of such fullerenes are impure which could be processed further to obtain highly pure fullerene. Yadav and Fulekar also reported the presence and recovery of fullerenes from the fly ash [12].
Sugarcane bagasse (SB) [123] is an agricultural waste that is rich in carbon content. Every year it is produced in MTs around the whole world and challenges a potential threat to the environment. The recovery of nanocarbons and GO from such waste will reduce pollution from the environment. The recovery of GO from sugarcane bagasse includes the following steps, collection of the fiber, crushing followed by grinding to obtain a powder, repetition of these two steps in order to increase the fineness of the powder [124]. Grounded SB and ferrocene were mixed in a 5:1 ratio by weight, in a crucible, and calcination was performed in a muffle furnace at 300 °C for 10 min under atmospheric conditions. The as-produced black solid was collected and the final product was analyzed.
One of the most systematically studied nanostructures, carbon nanotubes (CNTs) are cylindrically shaped materials with lengths in the order of few microns while the cross-sectional diameters are in the nanometer range [125][126]. The elongated surface of these materials makes them robustly versatile for their functionalization has driven need-based applications. Although the hybridization of constitutive carbon atoms is sp2 (similar to graphene), the arrangement of atoms is relatively distinct (that does not form layers). The two known variations are single-walled and multiwalled, with a high purity and cost of the former. The most extraordinary feature of these materials is their structural toughness, imparted by inherently high rigidity, Young’s Modulus, coefficient of elasticity which together are the reasons for their robust suitability in civil, defense, aeronautic and many other strategic applications [126][127]. It is because of such remarkable properties that these materials are widely preferred for developing immobilization-based assays, with high detection sensitivities. An interesting aspect of these nanomaterials is that based on their geometry and chiral carbon vicinity, these can be electrically conductive, semi-conductive, or insulated [126]. These adjustable electronic properties form the basis of their usage in single electronic transistors, flexible automated diodes where electron flow needs to be manipulated [128]. Comprised of only carbon, a variety of substrates have been used to obtain nanotubes via differently explored mechanisms. The most widely used methods of preparation are laser ablation, CVD [129], and electric discharge, which necessitate the provision of a specific stoichiometric mix of precursors. Though there are some concerns regarding the drug delivery application of these materials (with a potential risk of toxicity initiation), still the ability of functionalization has minimized such concerns and enabled a dose and location-specific drug delivery with them. Readers are suggested to consider more specific literature sources regarding the biological applications of these nanomaterials. Substrates as common as biscuits, chocolates, waste tyres, rubber, and manifold carbon-comprising substances have been used to prepare carbon nanotubes [130][131][132].
Several studies report the preparation of CNTs from fly ash, with a 2016 study claimed the utility and aptness of Saudi Arabian fly ash to provide CNTs using chemical vapor deposition method, provided all reaction conditions are maintained [133]. The preparation of CNTs from fly ash could be considered as an alternative to the famous electric arc-discharge method, with significant reports of transition metals (Mn, Mg, Ca, Na, Pb, Cd, Cr, Co, Ni, Zn, and Mo), present as traces in the fly ash. Depending on the regional geography and parent source of generation, the transition metal composition and diversity extents may vary amongst different sources. A generalized idea of typical fly ash composition is mentioned in Table 1. This synthesis of CNTs serves a dual purpose, one being the minimization of hazardous waste in the environment, the other being the cost-effectiveness and minimized use of energy. So, this approach is fittingly a green solution to minimize the undesired environmental risks of fly-ash by means of a sustainable approach. Research on particulate matter pollution does pose a concern of significant respiratory complications from inhaling fly-ash.
Table 1. Elemental composition of fly ashes.
| Elements | Composition (wt. %) |
|---|---|
| SiO2 | 40–60% |
| Al2O3 | 20–40% |
| Fe2O3-Fe3O4 | 5–15% |
| TiO2 | 2–5% |
| Carbon | 5–20% |
| CaO, BaO, MgO, MnO, P2O5 | Traces |
Carbon based everyday gadgets, such as plastic materials, tyres [75], rubber end products, and several other forms, can be readily used for making CNTs, using several modifications in their subsequent chemical treatment approaches. The generation of plastic wastes to the tune of billion tones on an everyday basis is one of the most pulsating concerns since plastic wastes often encounter a disposal problem due to their biodegradability concerns. Plastics are viciously produced as waste products from industries, household routines, laboratories, hospitals and eateries. Although the non-biodegradable nature of these materials has resulted in their substantial recycling, recycled plastics often lose their plasticity. Many studies have nevertheless used the plasticity intact waste materials to make CNTs via processing under varying oxygen environments. In one such study, plastic waste was readily decomposed to propylene which subsequently catalyzed the MWCNT formation over the surface of metal catalysts [134]. The growth mechanism is well known, reportedly following a tip-growth or base-growth pattern in the course of a vapor–liquid–solid reaction [135][136]. Nevertheless, there is still no clarity regarding the utilization of carbon atoms whether in the bulk catalyst or react within the top surface of the catalyst. The reaction was mediated by the utilization of reactor material (SS 316 tube of a CVD reactor), with the confirmatory studies revealing that removal of Cr from the reactor vessel resulted in MWCNT growth. Similar studies on SS 316 mesh surface found the involvement of Fe and Ni in the CNT formation. The results were in agreement with the works by Levendis and co-workers with a further ensuring of metal catalyst prevalence along the tip of MWCNTs inside the tubes [137].
Similarly, a 2016 study by Zhang and Williams reports the synthesis of MWCNTs along with hydrogen generation by the catalytic pyrolysis of waste tyres. The study employed a catalyst system comprising of a Ni/Al2O3 prepared via impregnation of Ni on the Al2O3 surface. The experimental procedure was optimized by varying the temperature from (700 to 900) °C, alongside varying the tyre to catalyst ratios from 1:0.5 to 1:1 and 1:2 and using steam input via injection of hot water at 0.2 and 5 mL per hour injection rates. Estimation of the carbon fractions (formed as product) revealed 253.7 mg per gram tyre to be comprising of filamentous carbons at 1:1 tyre to catalyst ratios at a catalyst temperature of 900 °C. Microscopic screening of the product showed a significant proportion of deposited filamentous carbons as MWCNTs. The procedure also released hydrogen at compatible rates that met the fuel and energy scarcity, making this overall approach a reliable and efficient methodology to utilize the tyre waste. An important aspect of this approach was that it firstly processed the nickel nitrate as nickel precursor by its dissolution in ethanol on alumina support that gradually converted into slurry via continuous stirring. The final catalyst was prepared on overnight drying of this slurry at 90 °C in an oven, at 2 °C per minute till the temperature reached 750 °C. This process took nearly 3 h of holding time, following which the solid material collected was crushed into (0.05–0.18) mm sized granules. It is interesting to note that the smaller size of catalyst particles conferred a higher surface area to the reacting species, so whether a different physical form of the particles would be able to provide the product in the same morphology with a similar yield remains a significant concern [132].
Quite recently, the synthesis of CNTs was reported from waste rubber-based substrates, with the experimental procedure utilizing the blended form of acrylonitrile butadiene and styrene-butadiene rubbers (NBR and SBR). The blend could not be conventionally decomposed due to its stronger mechanical strength and thermal resistance, however, the pyrolysis of the disposable form of the blend was optimized at 450 °C in a horizontal CVD pyrolyzer with a cautiously maintained nitrogen supply to yield hydrocarbon fractions. Upon allowing the CVD of these hydrocarbon fractions on different catalytic systems at 850 °C for half an hour, the screening of formed product using HRTEM, thermo gravimetric analysis (TGA), and Raman spectroscopy inferred a significant formation of SWCNTs to an efficient extent. Subsequently, in the course of physical analysis, it was noted that adjusting the crystallinity of Fe-Ni catalyst on different zeolites was a critical factor affecting the structure and diameter of as formed CNTs [75]. So, approaches like these are all potential solutions to synthesize nanotubes in desired yields from robust, cheap, and biocompatible materials ensuring, minimal pollution risk and higher output yields compared to costly and energy intensive conventional methods.
In the different parts of the world, rice husk (RH) shows as one of the most dominant crop residues and the disposal of which often results in crucial environmental risks [138][139][140]. The major constituent of RH as well as its burnt ash is silica (up to 90%) (widely used as fillers and area enhancement specific applications). So, efforts to utilize RH in its native as well burnt forms as a reliable material providing energy are on a rapid high. Furthermore, the global RH production registered a nearly 6% increase from 2010 to 2014, which represent an alarming threat as an environmental hazard [141]. The utilization of rice husk (substantially comprising carbon, nitrogen, and hydrogen) commences with gasification (or pyrolysis), which generates fragments suitable for power generation and biologically compatible charcoal. The one deemed fit for power generation could be utilized as such via landfilling and fertilizer application. However, the fraction acting as bio-reduced char contributes significantly to industrial activities. This fraction provides three potential materials, active carbon, porous carbon and amorphous silica, all of which have highly good absorption characteristics conferred by their significant surface area contributions. While amorphous silica finds peculiar suitability in soil improvement and the cement industry the active and porous carbon fractions are highly efficient adsorbents and used for wastewater treatment applications. So, with a carbon texture, the normally waste RH could be potentiated into manifold useful industrial products. Readers can have a detailed look at the RH utilization and processing methodology in a highly informative contribution made by Nguyen et al. in 2019. This is a review article that comprehensively discusses the engineering and industrial potential of RH and its derivative fractions (such as silicon nitride, magnesium silicide and others) as refractory materials, filler agents in thermoplastics, as reinforcement agent, the adsorbent in polymer composites and many others.
Osman et al. reported the synthesis of activated carbon and CNTs by using Miscanthus × giganteus which is a high silicate containing common perennial grass. Both the particles were characterized by the sophisticated instrument, i.e., mainly for their pore size prior to their application in wastewater treatment [142].
Oils represent some of the most used commodities which are basically natural hydrocarbon precursors having varying carbon chain fatty acids. The carbon skeleton of oils, accompanied by a range of physical and chemical modifying technologies such as fractional crystallization, fractional distillation, chromatographic separation, aqueous two-phase attraction are the incentives for their reduction procedures that could enable a range of products. Several kinds of oils, such as turpentine, eucalyptus, palm, turpentine, neem (Azadirachta indica), and sunflower, have been reported to enable efficient scale synthesis yields of CNTs and graphene [143]. The use of turpentine oil in the making of CNTs has been proposed by Chatterjee et al. through its decomposition on the surface of finely dispersed Co catalyst at 675 °C optimized the CVD method to synthesize CNTs. The study also proposed the application of synthesized CNTs in making efficient electrochemical double-layer capacitor [144].
In several interesting modifications, scientists have optimized the use of neem, sunflower, sesame, camphor and castor oils as the parent carbon sources for CNT synthesis. The utilization of sesame oil has attracted significant scientific attention, owing to its edible nature, clean methodology and formation of hollow CNTs with diverse shapes and morphology [145]. The formed nanotubes had no Fe nanoparticles in the interior, had diameters within 50–60 nm, and a sheet-like structure showing an intricate long-range array of folds. Thus, synthesis of nanotubes from oil represents the renewable, energy-efficient, cost-effective and most importantly, much more compatible to environment and laboratory personnel [146]. So, since the CNTs inception, making CNTs in big yields is now no more a herculean task than in the beginning years.
Poultry products or waste are also rigorous sources of carbon materials and their derivatives and are mostly comprised of carbohydrates and proteins, along with a dense supplement in the form of calcium [147]. Regarding the utilization of these materials to meet the energy concerns, eggshell material promises to be a very rich source of providing carbon skeleton, it has been used with significant interest to optimize the microbial growth for designated yields of biofuels. Though CNTs are concretely not reported as being synthesized from these materials, yet a modified version, namely, C-dots (inherently carbon comprising quantum dots) have successfully been synthesized using this natural resource. The primary advantage of these nanomaterials compared to conventional quantum dots is their low toxicity. A 2012 study reported from China has optimized the microwave assisted approaches (providing intensive and efficient energy) to process egg shell material for a reduced reaction time to obtain C-dots [148]. The study aimed at the microwave treatment of eggshell material to form C-dots, having a maximum fluorescence peak (at 450 nm) alongside a quantum yield of ~14%. The modification of operational parameters like reaction time (microwave duration), temperature, and the relative contents of eggshell material could be the significant leads to obtaining many other variations in the products, and for obtaining the biologically and biophysically more robust product designs.
These compounds are the new class of materials, which have remarkable mechanical properties which can be tuned very easily in order to dope with numerous dopants. Cecilia et al. reported the synthesis of Fullerene like sulfocarbide studied with difunctional theory (DFT). They studied their formation, energetic and structural effects of sulphur atoms at carbon sites in a graphene-like network and many other properties [36]. FL like CPx was also reported by Furlan et al., in 2006 and studied the relative stability of precursors and defect energetics during synthetic growth [149].
The synthesis of 2D nanoparticles from agricultural and industrial waste not only makes the product economical, but it also minimizes the solid waste pollution arising every year. Government organizations expands a large amount of money on the handling of such waste. So, synthesis of nanoparticles by using all the above industrial and agricultural waste makes an ecofriendly and economical approach. However, their acceptability among industries can be drastically enhanced by either surface modification or by isomerization. Both steps will bring a change to the external surface of 2D nanoparticles. One of the most common 2D nanoparticles which can be easily surface functionalize is graphene. The surface modification of GO can be carried out by several methods for instance; modification by means of chemical, covalent and non-covalent modification, electrochemical modification, and decoration of graphene by metal and metal oxide nanoparticles [13]. All these surface functionalization techniques makes the graphene specific for instance in biomedical, wastewater treatment and agricultural applications [150]. For the elimination of any specific pollutant from the wastewater, graphene can be modified, which will enhance the specificity of the graphene [151]. Similarly, in the medical field, such graphene can also be decorated with the antibacterial nanoparticles, e.g., Ag, ZnO, etc., which will have effective and efficient antimicrobial effects [152]. By surface modification and isomerization, the applicability of the 2D nanoparticles like graphene could be increased for industrial and daily purposes.
Carbon-based 3D nanomaterials are very promising for various applications, i.e., electrochemical energy conversion and storage etc. Various carbon allotropes doped heteroatoms can be utilized for low-cost mass production of electrode materials. Porous 3D carbon provides multiple advantages, such as large surface area, maximized exposure to active sites, 3D conductive pathways for efficient electron transport, and porous channels to facilitate electrolyte diffusion. It is difficult to synthesize and functionalize isotropic 3D carbon structures still very useful material for various applications. There are several wastes generated from biological activities like agriculture, or from the industry, e.g., red mud, fly ash, etc., which could be used as a precursor material for the synthesis of 3D carbon nanomaterials. There is also incense sticks ash, produced after burning incense sticks at houses and temples in South Asian countries. Since such incense sticks are ignited at low temperature, about 20–30% ash has unburned carbon, which could be processed further for the synthesis of an economical and sustainable source of carbon nanomaterials [153]. Paul et al. reported the synthesis of highly porous 3D CNT foam as an anode for the Li-ion based batteries for energy-based applications [154]. Paul et al. reported the synthesis of 3D heteroatom doped CNMs, as multifunctional ferrous free catalyst for the applications of enery storage devices [155]. Paul et al. synthesized BN co-doped CNT based nanoporous brushes for energy-based applications especially in the form of supercapacitors at elevated temperatures [156]. Maria et al. emphasized the importance of polymers in the design of 3D CNTs-based scaffolds for biomedical purposes [7][157].
The speciality of nanomaterials lies in their tunable nanoscale dimensionality, on the basis of which these are considered as one, two or three dimensional [158]. Thus, two-dimensional nanomaterials are typically those materials that have two of their three dimensions restricted to <100 nm [159]. There is no clear consensus regarding the upper limit of this restriction. This implies that, in these materials, it is feasible to retrieve the quantum scale effects on two dimensions, i.e., the restriction of the electronic motions of excited state electrons (more conventionally known by the terminology “quantum confinement”). Examples of these materials include nanosheets, fibrous networks having nanometric widths and heights with lengths in the order of micrometres. Popular applications of these materials include their inclusion as catalysts, electronic/battery devices, hydrogen sensing, laser protection, magnetic memory devices, and other domains, based on surface plasmon resonance (SPR) attributes [160].
In the present-day energy-savvy scenario, everyone is anxious to obtain quicker and greater product formation, minimizing not only the operational steps, but also the energy requirements. 2D NMs serve as ideal solutions to all these concerns in having a high aspect ratio, high electron mobility, unsaturated surface coordination, and unique material properties (especially physical, chemical and electronic) [161]. The ultrafine thickness of these materials confers upon them ultrahigh specific surface areas and high surface energy, making them appropriate towards numerous surface-active applications such as those in fuel cells. For the efficient function of these cells, oxygen generation and transport have to take place at reasonably good rates. The catalytic approaches in most general cases employ platinum (Pt) nanoparticles (NPs) immobilized on the surface of the carbon substrate. However, due to their high costs and slow reaction kinetics, the use of Pt NPS is not economically as well as commercially viable. To tackle these issues, the development of new methods like alloying and nanostructured engineering which could ensure maximum activity, stability along with cost minimization has emerged to be a priority [109]. Amongst the several different shapes attainable by noble metal alloys, ultrathin 2D sheet-like structures having a single or few atoms thickness are acquiring significant interest because of their large size, high electron mobility and surface energy. These features confer a high surface area to volume ratio to the ultrathin 2D sheet-like materials thereby giving rise to a high density of unsaturated atoms. For instance, Hong et al. have reported faster ethanol oxidation using ultrathin free-standing Pd-Pt-Ag (ternary) noble metal alloy [162]. Similarly, Din et al. proposed the suitability of quaternary noble metal alloy Pt-Cu-Bi-Mn (porous nanosheets) having 3–4 nm thickness as novel catalysts having high oxygen (reduction and oxidation) capabilities apart from a significant methanol tolerance [163]. Paul et al., 2019, highlighted the importance of carbon NMs based metal-free electrocatalyts for various applications [164]. Further, Paul et al. reported the applications of nanoporous graphitic carbon for supercapacitors and similar purposes [165]. Cheng et al. reported the 3D printing functional NMs for the electrochemical and energy storage purposes [166].
Bismuth-based nanomaterials are a unique category of materials that holds interesting properties like chemical, electrical and catalytic activities. Bismuth-based nanoparticles, including bismuth chalcogenides, bismuth vanadate, and bismuth oxyhalides, continue to show excellent photocatalytic activity in wastewater treatment. Bi-based materials like halogen combine Bismuth materials are showing very good topological applications. Freitas et al., 2015 synthesized 2D-Bi containing single layers which were preserved by hydrogenation and concluded that the hydrogenation step, provides a flexible chemical tenability that has the potential to preserve the band topology of the pristine XBi phases [167].
Sial et al., have rigorously compiled the several methods of making nanosheets (NSs) and their limitations in the present scenario (pertaining to energy considerations and economic constraints). Different methods of synthesizing 2D NSs are carbon monoxide (CO) confined growth, hydrothermal/solvothermal synthesis, wet chemical synthesis, self-assembly of NPs, topochemical reduction method, template-based synthesis, seeded growth, and microwave-assisted growth. Even though each of the methods provides specific characteristics of products in terms of morphology, the unanimous factors affecting their implementation are the need for robust catalysis (which offers lesser reaction time and is less costly) and the requirement of energy from an external agency. For example, CO assisted growth method allows the preferential growth on the substrate due to good surface adsorption of CO. These methods are workable through the feasibility of interactional distinctions of water and non-aqueous solvents, such as viscosity and dissociation constant. The process is characterized by selective oxidative etching enabling attainment of specific anisotropic growth. Two critical requirements of these methods are optimum reaction temperature maintenance alongside the steady action of a reducing agent. Likewise, wet chemical synthesis offers layered patterns of ultrathin NSs, with industrially scalable products allowing no CO requirement (unlike the CO assisted growth method and hydrothermal/solvothermal method). Another mechanism of interest is self-assembly which provides NSs regulated by weak binding interactions and comparatively larger sizes. However, the advantage in this method is that requirement of energy from the external end is very low and the constituent species themselves acquire a minimum energy configuration. Like-wise, the topochemical reduction approach is specifically suited for making single crystalline metal alloy NS utilizing Ni and Co as a combined catalyst in an aqueous medium while the template synthesis method is an efficient strategy to obtain layered nanostructures and extensively utilizes graphene and its derivatives as templates. Comparing the basic requirements of these two methods, it is quite evident that the template synthetic approach offers much higher control with every successive step being regulated by the chemical composition of the preceding deposited material layer. Another benign approach for making 2D NSs is the use of microwave technology, which is especially preferred for making inorganic nanomaterials having high quantum yield and high precision. Although this is a green approach, it is yet again dependent on energy input from outside. Often template-based synthesis mechanisms utilizing hydrothermally fabricated catalysts have relied on for commercial purposes [168].
The working of fuel cell involves rigorous electrochemical processes, characterized by an electrocatalytic oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER), involving formic acid oxidation and alcoholic oxidation at cathode and anode. The major problems encountered in the commercialized application of fuel cells are improvements in the electrode preparation with minimized use of precious metals, controlling the kinetics of the electrochemical process which collectively reduces the output efficiency of a fuel cell. So, in general, faster, more efficient, and rigorous catalysis with minimized expenditure and care requirements are the key. With continuous better understanding, several alternative mechanisms have emerged as steady sources of energy provision, like microbial driven fuel cells which utilize the energy generated from microbial metabolism (the functioning of enzymes and key pathways). However, this recourse is also not free of constraints as there is a constant need to ensure optimum microbial activities through providing specific pH, temperature, humidity and minimizing the ion concentration [169]. Recently, a new methodology making use of CNT-based composite materials has emerged. The concurrent hindrances related to dependence on water for conductivity, high methanol permeability, frequent disintegration (of conventionally used materials) in the presence of –OH radicals and low to moderate chemical stability have been the reasons to screen a safer, more reliable and efficient alternative. A novel attempt in this direction has been the use of nafion based membranes and its composite with the inclusion of CNTs as polymer electrolyte material (PEM) has provided a solution to recurrent limitations, through its greater mechanical stability, greater tensile strength and stronger physical texture [170]. Thus, nanomaterials provide numerous structural benefits to improve the fuel cell working through improvement in catalysis and energy-savvy functioning.
SPR is the characteristic phenomenon driven by the dominant surface effect of nano-materials, and more specifically the metal or metal oxide NPs. These entities absorb light at maximum at a peculiar wavelength after which the constituent ions are excited and progressively move to a high energy state. As the temperature increases (due to the input heat or light energy or via intermolecular frictional activities), these excited particles rapidly move with a net charge and remain in the semi-solid state, termed as plasma. The terminology plasmon is originated from the essence of ions existing in the plasmonic state. The resonance implies an instant where the light energy absorption is maximum, owing to which the manifested surface effects are also greater. Each nanoparticle has a characteristic SPR corresponding to a particular kind of incident light, so the SPR wavelengths are often used as identifiers for the formation of specific NPS. Since there is maximum energy absorbance in the SPR event, the nanoscale effects are also highest at this particular instant, giving rise to maximum bioactivities or quantum confinement dependent properties. The applications of nanomaterials have been significantly improved after a clear understanding of this phenomenon, with bulk species or sensing moieties being swiftly re-placed either by individual NPs (bound in membranes) or by the combination of nanomaterials (such as assembled nanostructures or hybrid NPs and thin layers of nanomaterials. For detailed insights into SPR and its consequent applications, readers are suggested to refer to more specific literature [171].
Probably, the cleanest, unanimously accessible and even most used form of energy, solar energy is a rigorous input agency for most of daily life activities. From microbes to plants, animals and even human beings, all require solar energy directly or indirectly for the sustenance of life. Commercial usage of solar energy presents exciting prospects, which are often limited by its low efficiency (substantially attributed to the uncertainty of availability) and inabilities to be scaled up. Lots of progress has been made via the use of nanomaterials in native and engineered form, to increase the absorption efficacy of the sun’s energy radiations. The most popular area has been the use of solar cell panels to provide electricity in which the functional circuit comprises an assembly of solar cells in a rectangular pattern. The efficiency of original assembly is quite low owing to which Si wafers (with amicable impurities) are added to it, which collectively not only improve the absorption but also manifold the utilization extents. Similarly, nanoscale attenuators and converters have been drafted into calculators to improve their charging efficiencies and performance. Lots of bioassays and drug carrier systems are in the market working through photothermal attributes of metallic NPs and their constitutive assemblies. Thin layers or assemblies of nanomaterials have emerged as carriers of more uniform and regulated solar energy absorption that remain localized to the surface and do not cause any serious effect in the bulk. Piezoelectric materials (such as MgO and ZnS based nanostructures) have come to the forefront, making use of pressure influences from solar energy (as input) to conduct the electricity or perform mechanical works. Many of these conceptualizations are in the research phase, with delays in optimization studies, meeting the scale-up regulations and constraints. Owing to this, the commercialization of such innovations is being delayed. Considering the energy crisis scenario (in particular for the developing world), these solutions could be potential remedies to eradicate the inadequate energy availability. Recently, the use of nanofluids (typically having either solid NPs or (1–100) nm-sized nanofibers suspended in a liquid) has been on the peculiar rise to enhance the utilization potential of solar energy [172]. These fluids, having dissolved nanomaterial(s), are able to enhance the outlet temperature by 30–100 K, enabling an enhanced potential to absorb the sunlight without any damage to native structures of the base material. One study claimed a more than 100% enhancement in photo thermal efficiency of 0.01% graphite-based nanofluid than without using it (normal functioning mode involving the coating of an absorbing collector). The use of these fluidic materials has enabled improved photovoltaic application via long-lasting existence in non-agglomerated form, having high stability without undergoing significant chemical changes in the base fluid [173]. The use of nanofluids has significantly improved the efficiency of electrolysis manifolds by the replacement of conventional electrolytes, allowing the faster and smoother conduct of chemical reactions [174].