1000/1000
Hot
Most Recent

Vitamin D is a secosteroid with a pleiotropic role in multiple physiological processes. Besides the well-known activity on bone homeostasis, recent studies suggested a peculiar role of vitamin D in different non-skeletal pathways, including a key role in the modulation of immune responses. Recent evidences demonstrated that vitamin D acts on innate and adaptative immunity and seems to exert an immunomodulating action on autoimmune diseases. Several studies demonstrated a relationship between vitamin D deficiency and autoimmune thyroid disorders. This topic review aims to summarize the evidences on the immunomodulatory effect of vitamin D on thyroid autoimmunity.
Autoimmune thyroid disorders (AITDs) are the most frequent autoimmune diseases with an estimated prevalence of 5% and a progressive increase in incidence, especially in the female population. Adult women have a higher risk of developing thyroid autoimmunity than men and present more frequently abnormal thyroid function in this context (7%–9% in females vs. 1%–2% in males)[1]. AITDs are T-cell mediated autoimmune disorders, resulting from an organ-specific deregulation of the immune system. The mechanisms involved in this autoimmune response have not been fully elucidated yet, though an interaction between genetic predisposition and environmental factors has been demonstrated to trigger the autoimmune process[2]. In subjects with genetic predisposition, an alteration of the physiological balance between Th1 and Th2 response may occur in case of exposure to environmental factors[3]. Moreover, a shift in the balance between Th17 and Treg cells has been recently observed in thyroid autoimmunity[4]. Environmental factors that have been recognized in association with AITD pathogenesis include iodine, radiation, smoking habit, viral infections, drugs, and stress[5].
The most common AITDs are HT and GD, which are commonly characterized by lymphocytic (T-cell CD4+ and CD8+) infiltration of the thyroid tissue and production of thyroid-specific antibodies[3][6] (Figure 1). Patients with AITD harbor an increase of activated T-cell expressing human leukocyte antigen (HLA)-DR and a decrease of CD8+ immune cells, whereas circulating B cell levels are normal[6]. The HLA-DR antigen, expressed primarily by monocytes and B cells, has also been detected on the surface of activated T cells. These DR antigens, which are cell-surface glycoproteins encoded by genes of the HLA-DR region of the MHC, are absent in resting T lymphocytes and could represent a potential marker of the immune system activation[7]. Some studies also documented that the percentage of circulating T cells expressing HLA-DR represent a biomarker capable of accurately reflecting autoimmune diseases activity[8].
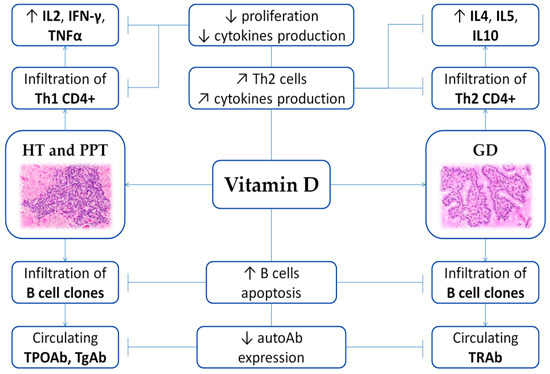
Figure 1. Scheme of the immunomodulating role of vitamin D on AITD. Arrows illustrate increase (↑), decrease (↓) or regulation/modulation (↗) of specific actions, processes, cells, or molecules. Abbreviations: autoAb, autoantibodies; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; IFN, Interferon; IL, Interleukin; PPT, Post-partum thyroiditis; Th, T helper; TNF, Tumor Necrosis Factor; TPOAb, anti-thyroid peroxidase antibodies; TgAb, anti-thyroglobulin antibodies; TRAb, TSH receptor autoantibodies. Histological images are available at Histology Gallery, Yale Medical Cell Biology.
As previously described, vitamin D exerts a modulating role on AITD through its specific enhancing effects on the innate immune system and inhibitory actions on the adaptive immune response[9]. Preclinical and clinical studies found an association between AITD and vitamin D deficiency[10][11]. Original evidence of a peculiar role of vitamin D in thyroid disease dates back to the late 80s to early 90s. McDonnell described an interesting homology between the VDR and the thyroid hormone receptor[12], and five years later, Berg et al. demonstrated the VDR expression on follicular thyroid cells[13]. Moreover, VDR and the thyroid hormone receptor share partners for heterodimerization[14]. In the same period, Fournier et al. investigated the effect of a combined treatment with cyclosporine A and 1,25(OH)2D3 using an experimental model of AITD in mice[15], suggesting a synergistic effect of these molecules in preventing the onset of thyroid autoimmunity and its associated histological alterations[15]. Years later, Borgogni and colleagues evaluated the effects of a non-hypercalcemic vitamin D receptor agonist, elocalcitol, on the secretion of the inflammatory chemokine CXCL10 induced by proinflammatory cytokines, as compared to methimazole. The authors demonstrated that, in human thyrocytes, elocalcitol impaired both IFN-γ and TNFα -induced CXCL10 protein intracellular pathways, whereas methimazole only aced on IFN-γ pathway. Moreover, elocalcitol reduced Th1 and Th17 cytokine secretion in CD4+ T cells and promoted a shift toward a Th2 response[16]. In murine models with induced autoimmune hyperthyroidism prompted by thyrotropin receptor immunization, hypovitaminosis D was found to induce a persistent disease, suggesting an immunomodulatory effect of vitamin D status on autoimmune hyperthyroidism[17]. In parallel, Liu and co-workers tested the effect of 1,25(OH)2D3 on Th1/Th2 cells and inflammation in female Wistar rats with experimental autoimmune thyroiditis[18]. Their results showed significantly decreased levels of thyroid autoantibodies and INF-γ in mice treated with 1,25(OH)2D3, which was associated with the maintenance of structural thyroid integrity.
From a clinical viewpoint, a meta-analysis including 20 case-control studies showed that patients with AITD harbor significantly lower serum vitamin D levels compared to healthy controls (OR 2.99, 95%CI 1.88–4.74)[19]. However, the mechanisms underlying the effects of vitamin D on AITD are still unknown but likely related to its anti-inflammatory and immunomodulatory properties.
HT represents a T-cell-mediated autoimmune disease characterized by goiter, presence of circulating anti-thyroid peroxidase (TPOAb) and/or anti-thyroglobulin (TgAb) antibodies, and intrathyroidal infiltration of B and T cells with a CD4+ Th1 predominance[2][20]. This alteration leads to varying degrees of thyroid hypofunction.
Observational and interventional studies observed that low vitamin D levels and the risk of HT onset seem to be closely associated. Indeed, patients with HT harbored a high proportion of hypovitaminosis D (over 60%). Moreover, HT is more closely related to vitamin D deficiency (<20 ng/mL) than insufficiency (21–29 ng/mL)[21][22][23][24]. The first observational study on the association between vitamin D and HT was published in 2009[25]. Based on the evidence that vitamin D deficiency is linked to a susceptibility to type 1 diabetes[26] and multiple sclerosis[27], Goswami et al. conducted a community-based survey on 642 adults to investigate the relationship between serum vitamin D concentrations and thyroid autoimmunity. Their results highlighted a significant inverse association between 25(OH)D3 and TPOAb levels[25]. Three years later, Camurdan et al. observed that hypovitaminosis D rate was higher in children with HT compared to control group (73.1% vs. 17.6%) and confirmed the inverse association between 25(OH)D3 levels and TPOAb titer in their pediatric population[28]. This inverse correlation was substantiated in following studies[23][29][30][31][32]. Furthermore, different clinical studies showed that the prevalence of HT in patients with hypovitaminosis D was significantly higher than that documented in subjects with sufficient vitamin D levels, particularly among children, elderly subjects, and pre-menopausal women[21][28][33][34][35][36][37][38]. As regards thyroid function in the context of HT, Mackawy and co-workers demonstrated a strong negative association between serum vitamin D concentrations and TSH levels, leading to speculate that vitamin D deficiency in HT patients could be associated with a progression towards hypothyroidism (TSH > 5.0 m UI/L)[22].
In more recent years, these evidences prompted several research groups to evaluate the effect of vitamin D supplementation on thyroid autoimmunity. Simsek et al. prospectively evaluated 82 patients with HT, which were randomized in two groups: the first group (46 patients) was treated with cholecalciferol 1,000 IU/day for one month and the second group without vitamin D replacement. Their results showed that TPOAb and TgAb levels were significantly decreased by the vitamin D replacement therapy in the first group[39]. These findings were confirmed by other prospective studies and randomized controlled trials, which added evidence that cholecalciferol supplementary treatment was related to a decrease in TPOAb and TgAb levels both in patients with vitamin D sufficiency and deficiency[40][41][42]. Moreover, an increase of 5 ng/mL in vitamin D levels was correlated to a significant decrease of 20% in the risk of HT[43]. In 2017, Mirhosseini et al. enrolled 11,017 subjects to evaluate the influence of vitamin D supplementary treatment on thyroid function and thyroid auto-antibodies levels. Their results showed that serum 25(OH)D3 levels ≥ 50 ng/mL were associated with a 30% decreased risk of hypothyroidism onset and a 32% decreased risk of increased thyroid auto-antibodies levels, leading the authors to speculate that vitamin D supplementation could exert a positive effect on thyroid function as well as provide protection from new onset of thyroid disease during a 12 months follow up[44]. In addition, in a recent 3 month randomized controlled trial (RCT) on adult females with HT, Chahardoli et al. confirmed a significant decrease of TSH levels after weekly supplementation with 50,000 IU of cholecalciferol[45].
Few studies, however, failed to document associations between vitamin D deficiency and a higher prevalence of HT[46][47], questioning on the preventive role of vitamin D in AITD. Further investigations are needed to evaluate the preventive and therapeutic effects of vitamin D in HT.
Growing evidence also documented that some VDR polymorphisms could be related to an increased incidence of HT[48]. The most frequent polymorphisms include FokI, BsmI, ApaI and TaqI. FokI polymorphism is located in exon 2 of the VDR gene and causes an alteration in the start codon leading to a truncated VDR protein[49]. The BsmI and ApaI polymorphisms, located in intron 8 of the VDR gene, lead to an altered mRNA stability, a disruption of splicing sites or a change in intronic sequences, affecting gene expression[49][50]. The TaqI polymorphism is located in exon 9 and is able to alter the mRNA stability[49][50]. FokI and ApaI polymorphisms influences serum vitamin D concentration, and BsmI polymorphism interferes with the IFN-γ production by monocytes, whereas TaqI influences the VDR expression[49][50]. In a meta-analysis on 8 studies showed that the VDR BsmI and TaqI polymorphisms were associated with HT risk[51]. Later, Inoue and co-workers demonstrated that the CC genotype for the FokI polymorphism was frequent in patients with HT[50]. Finally, a meta-analysis including 11 studies on Asian and Caucasian populations observed that the FokI polymorphism of VDR was related with a higher risk of HT only in Asian subjects[52]. All these results are in line with findings on children with type 1 diabetes[53].
GD is the most common cause of hyperthyroidism in developed countries, affecting mostly women, with an annual incidence of 14 cases in 100,000 persons[54]. GD is characterized by the presence of TSH receptor autoantibodies (TRAb) which lead to hyperthyroidism, diffuse toxic goiter, and ophthalmopathy[55]. In GD, infiltration of lymphocytes is milder than in HT and involves mainly CD4+ Th2 cells[2]. Although several studies reported an increased prevalence of hypovitaminosis D in patients with GD, the relationship between these two conditions is not clear[56].
The first observational study evaluated vitamin D status in women with and without GD remission. The results showed that vitamin D concentrations were significantly lower in patients without remission of GD compared to subjects with remission and that the prevalence of hypovitaminosis D was twice as high as in healthy controls[57]. The same workgroup, in a prospective study, observed a significant association between low vitamin D concentrations and an increased volume of thyroid gland in women with newly onset GD[58]. In 2016, Kim et al., in a cross-sectional study including 776 AITD patients, showed that the prevalence of vitamin D insufficiency was higher in GD patients compared to healthy subjects[36]. These results were further confirmed by two cross-sectional studies, although no association was observed between vitamin D and TRAb levels[59][60]. Conversely, in a cohort of 70 GD subjects, Zhang et al. found an inverse association between serum vitamin D concentrations and TRAb levels[61]. More extensively, Xu and co-workers evaluated the relationship between serum vitamin D levels and GD through a meta-analysis including 26 case-control or cohort studies. Their results confirmed that subjects with GD were more frequently to be deficient in vitamin D than the control group (OR = 2.24, 95% CI 1.31–3.81, p < 0.001)[62].
As regards the role of vitamin D supplementary treatment during GD, current evidence is limited to only one interventional study where the effect of daily vitamin D treatment was assessed on GD recurrence. Among 210 GD patients with hypovitaminosis D, 60 received cholecalciferol (1,000–2,000 IU per day) whereas 150 did not. Recurrence rate was comparable between groups (38% vs. 49%) but occurred earlier in the control group (7 vs. 5 months)[63].
Several studies investigated the relationship between polymorphisms of VDR gene and GD onset risk, but results remain arguable. The first meta-analysis to evaluate this association was conducted by Zhou et al. in 2009 and included seven studies on Caucasian and Asian populations. The results showed that the presence of ApaI, BsmI, and FokI VDR polymorphisms was associated with a higher risk of GD onset in Asian population, whereas no associations were found in Caucasian cohorts[64]. More recently, a meta-analysis including eight studies found a relationship between BsmI and TaqI polymorphisms and the risk of GD onset, while no correlation was seen for ApaI and FokI[51]. Finally, Inoue et al. observed a higher prevalence of TT genotype for TaqI in subjects with GD compared to patients with HT and a higher prevalence of the C allele for ApaI in comparison with controls[50].
Post-partum thyroiditis (PPT) refers to the development of de novo AITD within the first year post-partum and represents one of the most common autoimmune disorders in pregnancy, with an estimated prevalence between 1% and 17%[65]. Clinical symptoms include a thyrotoxic phase during the first 3 months of onset usually followed by a phase of hypothyroidism at 3–6 months, which is reversible in 75% of patients[66][67].
Different clinical studies investigated the relationship between PPT and serum vitamin D concentrations. Krysiak et al. compared 25(OH)D3 and PTH levels between 4 groups of non-lactating women who gave birth 12 months before the beginning of the study: euthyroid women with PPT, women with hypothyroidism and PPT, women with non-autoimmune hypothyroidism, and healthy euthyroid women without AITD. Serum vitamin D concentrations were lower whereas PTH levels were higher in patients with PPT compared to subjects without AITD. Moreover, in the second part of the study, women with hypothyroidism and PPT as well as women with non-autoimmune hypothyroidism were treated for 6 months with L-thyroxine. The results showed that L-thyroxine therapy increased serum vitamin D levels and reduced PTH levels only in the first group, highlighting an intriguing relationship between vitamin D status, PPT and L-thyroxine therapy[68]. In 2016, the same group investigated whether vitamin D treatment could modify the course of thyroid autoimmunity in 38 non-lactating levo-thyroxine-treated women with PPT compared to 21 matched healthy postpartum women. Women with deficiency of vitamin D were treated with oral cholecalciferol at 4000 IU daily, whereas women with insufficiency of vitamin D and women with normal 25(OH)D3 concentrations were either treated with cholecalciferol at 2,000 IU daily or left untreated. At baseline, serum vitamin D concentrations were lower in patients with PPT compared to healthy women and were inversely associated with thyroid antibody levels. Following vitamin D treatment, TPOAb titer decreased, and this effect was more evident in women with hypovitaminosis D compared to those with normal vitamin D[69]. However, this study raised some criticism regarding the presence of potential confounders that could interfere with autoantibody titer and the vitamin D status, including the use of estrogen contraceptives, iodine status, and selenium levels[70]. Further studies are needed to define the role of vitamin D in PPT.
In conclusion, several studies observed a relationship between hypovitaminosis D and thyroid autoimmunity. Supplementary treatment with cholecalciferol seems to have beneficial effects on AITD. However, large multicenter studies are needed to investigate the impact of vitamin D supplementary treatment on meaningful long-term clinical end points in these patients.