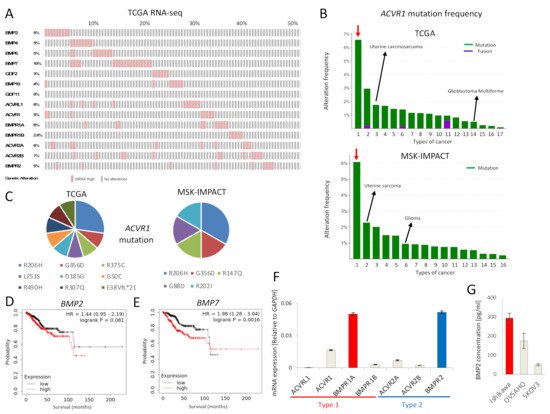
The expression of mRNA for BMP ligands and receptors was found to be frequently increased in EC, as revealed by analysis of the TCGA EC database (
Figure 1A). In addition,
ACVR1 mutations were more frequently observed in EC compared to other cancers, in both the TCGA and MSK-IMPACT datasets (
Figure 1B). Around half of
ACVR1 mutations were R206H and G356D (
Figure 1C), gain-of-function mutations commonly found in fibrodysplasia ossificans progressiva (FOP) and diffuse intrinsic pontine gliomas (DIPGs)
[5]. Moreover, high expression of
BMP7 mRNA correlated with significantly lower survival of EC patients; the expression of
BMP2 mRNA also showed a correlation, albeit not significant, with poor EC patient survival (
Figure 1D,E). We also performed survival analyses of other BMP ligands and receptors (Figure S1). However, there was no correlation between
ACVR1 mRNA expression and EC patient survival. To investigate the tumor promoting effect of BMP signaling in EC, we further performed in vitro experiments using Ishikawa EC cells, revealing expression of mRNA for all type I and type II BMP receptors, except
ACVRL1 (
Figure 1F).
BMPR1A mRNA was most abundantly expressed among the type I receptors, whereas
BMPR2 mRNA was most abundant among the type II receptors (
Figure 1F). In addition, we found that Ishikawa cells secreted BMP2 at a higher level than OVSAHO and SKOV3 ovarian cancer cells, as determined by an ELISA (
Figure 1G).
Figure 1. BMP signaling is activated in EC. (A) mRNAs for BMP ligands or receptors are over-expressed in EC. RNA-seq of the TCGA endometrial cancer dataset containing 177 EC tumors was analyzed via cBioPortal. RNA expression cutoff Z score was adjusted to 2.0. The results of 90 tumors are shown. (B) ACVR1 is more frequently mutated in EC compared to other cancers. ACVR1 mutation frequency of the TCGA pancancer atlas studies and of the MSK-IMPACT clinical sequencing cohort was assessed by cBioPortal. Red arrows point to EC. Lanes 1 to 17 (in the top panel); endometrial carcinoma, skin cutaneous melanoma, uterine carcinosarcoma, colorectal adenocarcinoma, bladder urothelial carcinoma, lung adenocarcinoma, mesothelioma, stomach adenocarcinoma, adrenocortical carcinoma, head and neck squamous cell carcinoma, ovarian serous cystadenocarcinoma, lung squamous cell carcinoma, liver hepatocellular carcinoma, glioblastoma multiforme, kidney renal clear cell carcinoma, brain lower grade glioma and breast invasive carcinoma, Lanes 1 to 16 (in the bottom panel); endometrial cancer, uterine sarcoma, melanoma, mesothelioma, cancer of unknown primary, glioma, esophagogastric cancer, colorectal cancer, hepatobiliary cancer, head and neck cancer, mature B-cell neoplasms, germ cell tumor, non-small cell lung cancer, renal cell carcinoma, bladder cancer and breast cancer. (C) Details of ACVR1 mutations found in EC are shown. Eleven cases of the TCGA dataset (out of 244 cases) and six cases of the MSK-IMPACT dataset (out of 113 cases) had the ACVR1 mutations indicated. (D,E) Overall survival was analyzed using RNA-Seq data of KM plotter, which contained 542 EC patients. Patients were divided into two groups, i.e., above or below median mRNA expression. The effects of expression of BMP2 (D) and BMP7 (E) on the survival of EC patients, are shown. (F) mRNA expression levels of BMP receptors in Ishikawa EC cells, as determined by qRT-PCR and normalized relative to GAPDH. (G) BMP2 secretion by Ishikawa cells, and by OVSAHO and SKOV3 ovarian cancer cells for comparison. Confluent cell cultures were incubated in serum-free medium for 24 h; thereafter, the conditioned medium was analyzed for BMP2 by an ELISA. BMP2 concentration was normalized to 1 mg total protein in lysates. The results in panel F and G are shown as the mean ± SE.