1000/1000
Hot
Most Recent

p-Coumaric acid is a natural metabolite contained in many edible plants, and its antioxidant activities in reducing oxidative stress and inflammatory reactions have been demonstrated in various experimental models. p-Coumaric acid has an optimal structure to be a competitive inhibitor of tyrosinase that catalyzes key reactions in the melanin biosynthetic pathway. Experimental evidence supports this notion as it was found to be a more potent inhibitor of tyrosinase, especially toward human enzymes, than other well-known tyrosinase inhibitors such as arbutin and kojic acid. p-Coumaric acid inhibited melanin synthesis in murine melanoma cells, human epidermal melanocytes, and 3-dimensionally reconstituted human skin models. Ex vivo skin permeation experiments and in vivo efficacy tests for p-coumaric acid confirmed its efficient transdermal delivery and functional efficacy in reducing erythema development and skin pigmentation due to ultraviolet exposure. Human studies further supported its effectiveness in hypopigmentation and depigmentation. These findings suggest that p-coumaric acid has good potential to be used as a skin-lightening active ingredient in cosmetics.
p-Coumaric acid (4-hydroxycinnamic acid) is a phytochemical with multiple health benefits [1][2]. Its chemical structure is very similar to that of L-tyrosine, the natural substrate of tyrosinase involved in the cellular melanogenesis in melanocytes. Recently, p-coumaric acid was found to be a potent and selective inhibitor of human tyrosinase [3]. Its antimelanogenic effects have been demonstrated in various experimental settings including human studies [4]. Considering the need for natural skin lightening agents in cosmetics, it is of interest to scrutinize recent literature on the biological activities of p-coumaric acid. This review focused on the antimelanogenic properties of p-coumaric acid to extensively examine its potential as an active ingredient in cosmetics.
A variety of phenolic compounds found in the plant kingdom are a group of natural antioxidants with potential benefits to human health and beauty [1][2][5]. Phenolic compounds with reducing power and free radical scavenger activity may be helpful in prevention or alleviation of many chronic diseases caused by oxidative stress [6][7]. Coumaric acids are derivatives of cinnamic acid mono-hydroxylated at the phenyl group, and p-coumaric acid is the most abundant isoform. p‐Coumaric acid is found at significant levels in many fruits, vegetables, and cereals [8][9].
It is stated that p-coumaric acid is a relatively potent antioxidant, and a scavenger of reactive oxygen species (ROS) and free radicals [10][11]. Its antioxidant activity has been demonstrated in cultured endothelial cells exposed to high glucose and free fatty acid [12], in keratinocytes exposed to UV [13], and in lens epithelial cells exposed to hydrogen peroxide [14]. It also shows antimicrobial activity by disrupting bacterial cell membranes and intercalating the groove in bacterial genomic DNA [8][15]. Polymeric preparations containing p-coumaric acid showed antioxidant and antimicrobial properties, aiding in the regeneration process of wounded skin [16][17].
In animal models, p-coumaric acid decreased basal oxidative stress more effectively than vitamin E, as assessed by DNA damages in rat colonic mucosa [18]. It enhanced cardiac antioxidant capacity in rats by activating nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that regulates antioxidant response element (ARE)-mediated gene expression of downstream target genes, such as glutathione peroxidases [19].
p-Coumaric acid showed antiinflammatory effects in adjuvant-induced arthritic rats, reducing the levels of tumor necrosis factor-alpha (TNF-a) and macrophage phagocytic index, while increasing serum immunoglobulin levels [20]. It further attenuated hepatotoxicity due to alcohol or acetaminophen [21][22], pulmonary inflammation due to lipopolysaccharide or cigarette smoke [5][23], and cardiotoxicity due to arsenite or doxorubicin [24][25].
In addition, p-coumaric acid has also been shown to inhibit proliferation and migration of cancer cells and promote apoptotic cancer cell death, supporting its potential anticancer effects [26][27][28][29]. Its chemopreventive effects against colon cancer have been demonstrated in animal models, wherein p-coumaric acid reduced inflammatory reactions and increased antioxidant capacity [30][31].
In 1999, p-coumaric acid was identified as an active constituent of ginseng leaves that inhibited mushroom tyrosinase activity in vitro [32]. In later studies, dimeric coumaroyl amides such as N,N'-di-p-coumaroyl-1,3-diaminopropane and N,N'-di-p-coumaroyl-1,3-diaminoethane inhibited mushroom tyrosinase activity as compared to dimeric feruloyl amide derivatives [33][34][35].
A systematic assay using mushroom, murine, and human tyrosinase preparations revealed that p-coumaric acid is a very selective and potent inhibitor toward human and murine tyrosinases than toward mushroom tyrosinase [3]. p-Coumaric acid inhibited human and murine tyrosinases ~100 and ~10 times more strongly than kojic acid, respectively, although their inhibitory effects against mushroom tyrosinase were comparable [3].
In another study, using human tyrosinase expressed in human embryonic kidney 293 cells, p-coumaric acid was shown to be the most potent inhibitor of human tyrosinase among the various phenolic acids tested [36]. The concentrations of some phenolic acids required for 50% inhibition of the enzyme activity (IC50) were as follows: 3 μM p-coumaric acid, 120 μM p-methoxycinnamic acid, 200 μM cinnamic acid, 250 μM caffeic acid, and 750 μM ferulic acid. p-Coumaric acid was more active than m-coumaric acid (IC50, 270 μM), o-coumaric acid (IC50, 300 μM), and other tested compounds, indicating it has an optimized structure to be an effective human tyrosinase inhibitor.
On the basis of enzyme kinetics studies, p-coumaric acid was classified as a mixed type or competitive inhibitor of human tyrosinase depending on the substrates used- L-tyrosine or L-DOPA [3]. Given the structural resemblance to endogenous substrate L-tyrosine, p-coumaric acid might bind to and block the active site of the enzyme, preventing access to its substrates.
In 2004, Kubo et al. reported that methyl p-coumarate decreased melanin formation in B16 mouse melanoma cells whereas p-coumaric acid did not show this activity [37]. In later studies, p-coumaric acid inhibited melanin synthesis in B16F10 cells whereas ferulic acid showed rather melanogenic or cytotoxic effects [38]. In addition, methyl p-coumarate showed more potent inhibition of melanin synthesis compared to methyl ferulate [39].
In 2008, Park et al. tested the constituents of Rhodiola sachalinensis against melanin synthesis in B16F10 cells and observed that only p-coumaric acid inhibited melanin synthesis, whereas catechin, chlorogenic acid, and p-tyrosol did not show such an effect [40]. In their experiment, p-coumaric acid competitively inhibited tyrosinase catalytic activity but had no effect on CREB phosphorylation or tyrosinase protein expression [40]. An et al. identified p-coumaric acid as an active constituent of Sasa quelpaertensis that attenuated cellular melanin synthesis stimulated by a-MSH [41]. The authors showed that p-coumaric acid was more active than structurally similar caffeic acid and cinnamic acid. They further showed that p-coumaric acid decreased tyrosinase protein levels.
Although there are minor inconsistencies among study results, most evidence supports that p-coumaric acid can attenuate cellular melanogenesis. Indeed, the antimelanogenic effects of p-coumaric acid have been verified in later studies using human epidermal melanocytes [3, 36], and 3-dimensional human skin equivalents [42].
p-Coumaric acid did not significantly decrease the melanin levels of “unstimulated” B16/F10 cells [33][37], but its inhibitory effects on cellular melanogenesis was clearly observed in cells in the presence of a-MSH stimulation [41]. This may indicate that p-coumaric acid prevents “stimulated” new melanin synthesis rather than decreasing preexisting melanin.
As inferred from the in vitro studies, p-coumaric acid can reduce new melanin synthesis through direct inhibition of the catalytic activity of tyrosinase [3][40]. p-Coumaric acid more potently inhibited tyrosinase catalytic activity when L-tyrosine rather than L-DOPA was used as the substrate [3][40]. The structural similarity of p-coumaric acid to L-tyrosine suggests that the former may compete with the latter for the limited active sites on the tyrosinase enzyme.
The effect of p-coumaric acid on tyrosinase expression levels in cells is controversial. In some studies, p-coumaric acid attenuated the protein expression of tyrosinase stimulated by a-MSH [41], but other studies showed that CREB phosphorylation and tyrosinase expression were not affected by p-coumaric acid [40]. Interestingly, L-tyrosine is known to not only act as the substrate for tyrosinase enzyme but also play a hormone-like stimulatory role in tyrosinase gene expression. L-tyrosine enhances the binding capacity of the receptors for a-MSH [43], increasing tyrosinase gene expression [44][45][46]. It is tempting to speculate that the binding of L-tyrosine to the regulatory site on the MSH receptors may be prevented by structurally similar compounds such as p-coumaric acid. This could be an additional mechanism for the antimelanogenic effects of p-coumaric acid under certain.
Although p-coumaric acid is a small molecule (molar mass: 164) that may be advantageous for skin and cell membrane permeability, it has one carboxyl group that is deprotonated at neutral pH, making the compound negatively charged and decreasing cell membrane permeability. Upon direct treatment of mouse melanoma cells in vitro, p-coumaric acid showed weaker inhibition of melanin synthesis than methyl p-coumarate [37]. Although p-coumaric acid was a more potent inhibitor of human tyrosinase (IC50, 3 μM) than methyl p-coumarate (IC50, 30 μM), the former inhibited melanin synthesis less effectively than the latter in human epidermal melanocytes stimulated with L-tyrosine [47]. This phenomenon may be explained by lower cell membrane permeability of p-coumaric acid than methyl p-coumarate, as demonstrated in the assay using a hexadecane-filled membrane as a model of lipophilic cell membranes [47].
Excised porcine skin is a good model for the study of permeation of human skin, because they share similar histological and barrier properties [24]. Skin permeability of p-coumaric acid and methyl p-coumarate were compared using a vertical type simple diffusion device where excised porcine skin was placed between the donor and acceptor chambers [47]. p-Coumaric acid and methyl p-coumarate were separately applied in the form of semi-solid emulsion to the donor chamber, and aqueous medium in the acceptor chamber was used for the analysis of p-coumaric acid and methyl p-coumarate with high performance liquid chromatography. The results showed that p-coumaric acid can pass through the skin into the underneath aqueous media, whereas methyl p-coumarate was captured in lipophilic skin layers or transferred into the aqueous media only after being converted to p-coumarate [47]. Although a hydrophobic property of a molecule is needed to enter the lipophilic layer of the skin, a hydrophilic property is also needed for diffusion out of the skin into the aqueous medium [48]. Because p-coumaric acid is an amphiphilic compound that possesses both hydrophobic and hydrophilic properties at neutral pH, its transdermal delivery can be faster than methyl p-coumarate which is very hydrophobic.
Although the antimelanogenic effects of p-coumaric acid were observed in cultured mammalian melanocytic cells, experiments using the animal model of zebrafish indicated that p-coumaric acid was not as effective as other shikimic acid pathway compounds such as shikimic acid [49].
When a cream containing 1.5% p-coumaric acid was applied on the skin of SKH‐1 hairless mice, it attenuated UV-induced inflammatory responses as monitored by skin thickness and skin redness, compared to the animals treated with a control cream [50]. The effects of p-coumaric acid cream on UV-induced skin pigmentation were also examined in Hos:HRM-2 melanin-possessing hairless mice [47]. UV exposure of mice increased the a* values and decreased L* values, representing erythema and skin lightness, respectively. The UV-induced changes in a* and L* values were significantly reduced in mice pretreated with creams containing 1.5% p-coumaric acid or methyl p-coumarate than those pretreated with control cream.
In addition, p-coumaric acid cream was found to mitigate the UV-induced erythema and subsequent pigmentation in human skin [4]. These effects of p-coumaric acid cream were attributed to p-coumaric acid because control cream lacking p-coumaric acid did not show such effects. p-Coumaric acid cream showed a skin depigmenting effect when it was applied after human skin was fully tanned by UV [4]. Thus, the application of p-coumaric acid to skin, before or after sun exposure, would be beneficial in terms of mitigating UV-induced erythema and maintaining a lighter skin color.
p-Coumaric acid showed no significant cytotoxicity at the effective concentration range inhibiting cellular melanin synthesis [41]. In addition, no toxic effects were observed in animal experiments and human studies [4][47].
p-Coumaric acid is an excellent candidate for dual function cosmeceuticals that provide both antimelanogenic and UV-protection effects. Human epidermal melanocytes treated with p-coumaric acid before UV exposure showed significantly lesser cell death than the control cells exposed to UV or the cells treated with p-coumaric acid after UV exposure [3]. This phenomenon is considered melanin-independent, because p-coumaric acid attenuated the UV-induced cellular melanin synthesis regardless of whether it was added before or after UVB exposure.
UV exposure of the skin induces gene expression and activation of matrix metalloproteinases (MMPs), a family of peptidases that degrade the extracellular matrix protein, thereby causing remodeling of intradermal tissue and formation of thick wrinkles [51]. Stratifin released from epidermal keratinocytes shows a paracrine effect on dermal fibroblasts, stimulating fibroblastic MMP1 gene expression by a p38 MAP kinase-dependent mechanism [52][53]. In an in vitro study, p-coumaric acid lowered the levels of stratifin released from the epidermal keratinocytes exposed to UV [54]. The conditioned media from epidermal keratinocytes containing different levels of stratifin stimulated MMP1 expression in dermal fibroblasts to varying degrees, indicating that p-coumaric acid indirectly reduced MMP1 expression in dermal fibroblasts by down-regulating stratifin expression in epidermal keratinocytes exposed to UV.
Urocanic acid is biosynthesized from L-histidine by the action of L-histidine ammonia lyase (also called histidase) and it is found as a major acid-soluble, UV-absorbing compound in the stratum corneum [55][56]. Urocanic acid is considered as a “natural sunscreen” having controversial effects on skin health [57-59]. p-Coumaric acid is similar to urocanic acid, in that it is synthesized from L-tyrosine, another aromatic amino acid, in a reaction catalyzed by L-tyrosine ammonia lyase in prokaryotes, plants, and animals [60][61]. p-Coumaric acid rescued the viability of HaCaT keratinocytes exposed to UV as effectively as urocanic acid in vitro [50]. Topical application of p-coumaric acid onto the dorsal skin of Hos:HRM-2 melanin-possessing hairless mice or SKH-1 hairless mice attenuated the inflammatory erythema responses caused by UV [47][50]. Pre-application of p-coumaric acid on human skin attenuated erythema due to UV exposure [4].
In conclusion, p-coumaric acid has a unique chemical structure, and many of its biochemical properties are suitable for its use as a skin lightening cosmetic ingredient. p-Coumaric acid inhibited the catalytic activity of tyrosinase in vitro, especially toward human tyrosinase, more effectively than other structurally similar compounds, especially when L-tyrosine was used as the substrate. p-Coumaric acid inhibited tyrosinase gene expression stimulated by a-MSH. Antimelanogenic effects of p-coumaric acid were observed in murine melanoma cells, human epidermal melanocytes, and 3-dimensional human skin equivalents. p-Coumaric acid also attenuated UV-induced cytotoxicity. Its skin permeability and hypopigmenting effects were shown in ex vivo and in vivo experiments, respectively. The clinical outcome from human studies was also supportive for the efficacy of p-coumaric acid attenuating UV-induced inflammation and subsequent pigmentation. Therefore, the antimelanogenic effects of p-coumaric acid in the UV-exposed skin is considered to involve multiple mechanisms: (1) absorption of UV, (2) inhibition of new synthesis of tyrosinase, and (3) inhibition of catalytic activity of preexisting tyrosinase (Figure 1).
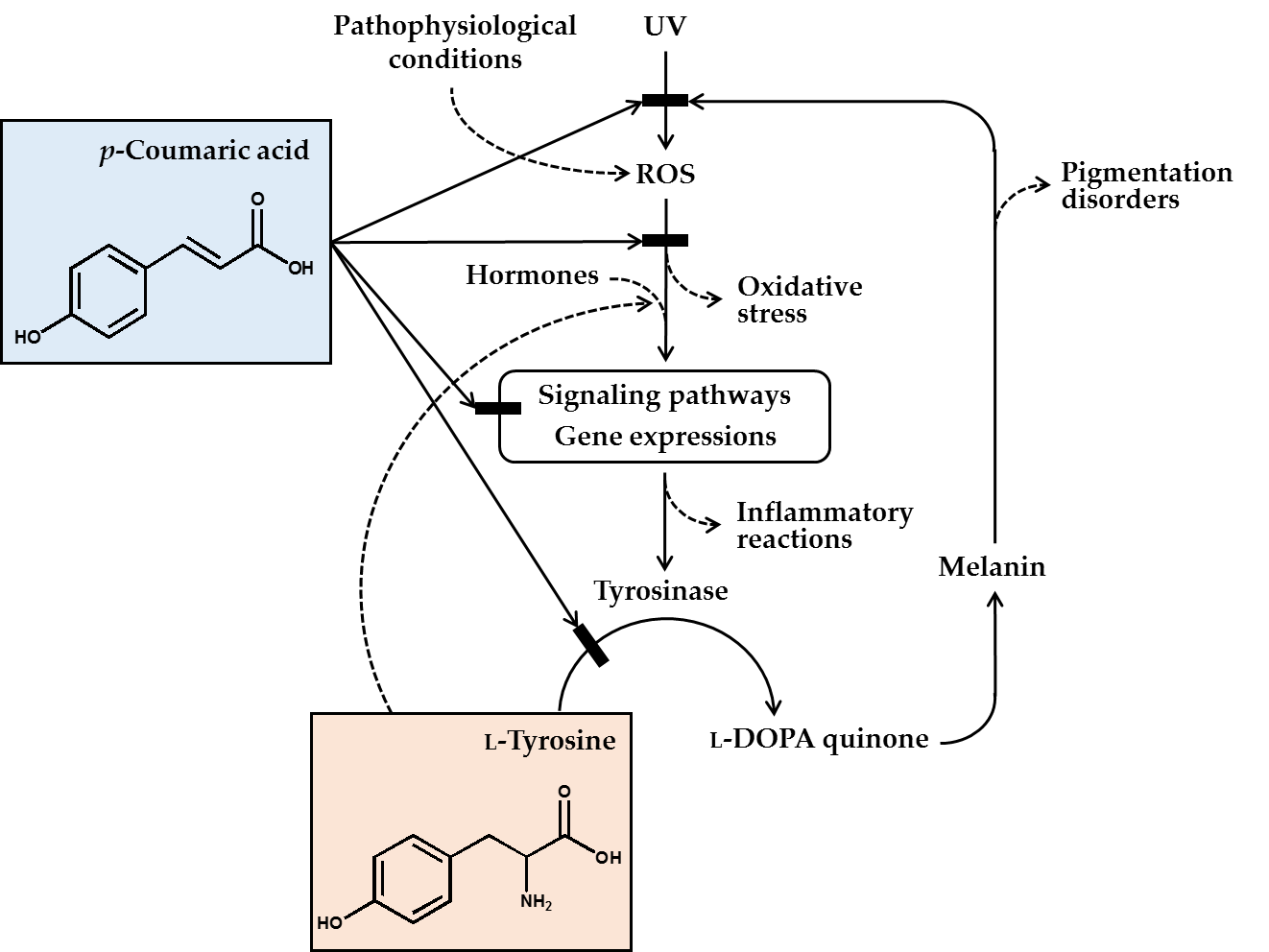
Figure 1. p-Coumaric acid can attenuate skin hyperpigmentation through multiple mechanisms. UV and other pathophysiological conditions stimulate the production of reactive oxygen species (ROS) and multiple signaling pathways leading to enhanced gene expression of tyrosinase and increased melanin synthesis. The melanin can absorb ultraviolet (UV) radiation and alleviate oxidative stress and inflammatory reactions caused by UV radiation, but the melanin deposition may cause skin pigmentation disorders. p-Coumaric acid has a chemical structure similar to L-tyrosine and inhibits the activity of tyrosinase, which catalyzes the oxidation of L-tyrosine and/or L-DOPA to L-DOPA quinone in the melanin biosynthetic pathway. Due to its UV absorption and antioxidant action, p-coumaric acid can inhibit the signaling pathways linked to gene expression of tyrosinase and inflammatory mediators. p-Coumaric acid can also reduce the stimulatory effects of hormones and L-tyrosine on the gene expression of tyrosinase. Thus, it is proposed that p-coumaric acid has advantageous biochemical properties suitable for use as a skin-lightening active ingredient in cosmetics.