1000/1000
Hot
Most Recent

Researchers present an overview of the current state of knowledge on the SARS-CoV-2 and COVID-19 pandemic. In addition to an overview of the epidemiological, clinical, and radiological features of SARS-CoV-2, researchers also summarize possible therapeutic options currently under investigation and the future outlook for the disease. Whereas the trials on SARS-CoV-2 genome-based specific vaccines and therapeutic antibodies are currently being tested, this solution is more long-term, as they require thorough testing of their safety. On the other hand, the repurposing of the existing therapeutic agents previously designed for other virus infections and pathologies happens to be the only practical approach as a rapid response measure to the emergent pandemic. The current pandemic emergency will be a trigger for more systematic drug repurposing design approaches based on big data analysis. Further on, regression analytical review is presented on the virological and evolutionary history of SARS-CoV viruses, indicating to the autoimmune pathogen.
The usual symptoms of COVID-19 include fever (83–98%), cough (59–82%), shortness of breath (19–55%), and muscle ache (11–44%), which are similar to those of SARS and MERS. [1] Some patients may have sore throat, rhinorrhea, headache and confusion a few days before the onset of fever, indicating that fever is a critical symptom, but not the only initial manifestation of infection. [1] The pattern of fever has not yet been fully understood. A small proportion of patients had hemoptysis [2][3], and a number of cases were found relatively asymptomatic. [4] COVID-19 patients may have normal or lower white blood cell counts, lymphopenia, or thrombocytopenia, with the increased C-reactive protein level. [1][2][3] People who have fever and upper respiratory tract symptoms with leukopenia or lymphopenia should be suspected for this disease, especially for patients with travel history to the endemic area or close exposure record.
However, the clinical course of COVID-19 pneumonia exhibits a broad spectrum of severity and progression patterns. In some patients, dyspnea develops within a median of 8 days after the onset of illness (range of 5–13 days), while in others, respiratory distress may be absent. [2] Around 3–29% patients may need the admission to the intensive care unit. Severely ill patients may have poor disease course of rapid progression to multiple organ dysfunction and even death [1][2], and those who have shortness of breath and hypoxemia can quickly progress into acute respiratory distress syndrome (ARDS), severe sepsis with shock, and even multiple organ dysfunction within one week. [3][5] ARDS was observed to develop in 17–29% of hospitalized patients approximately 8 days after symptoms onset, and the global mortality rate reached approximately 5.4% [2].
It is also worth noting that the gastrointestinal symptoms of COVID-19 may be caused by the direct viral damage to the intestine rather than the immunopathogenic response to the lung infection of the host. Since angiotensin-converting enzyme 2 (ACE2), the main cellular receptor of SARS-CoV-2 is expressed in the human gastrointestinal epithelial cells, it is believed that the viral shedding at the gastrointestinal tract and fecal–oral transmission is highly plausible. [6] Indeed, it was reported that the rectal swabs showed positive results even after the nasopharyngeal tests were constitutively negative [7]. Besides, the live virus was also detected in stool samples of diseased patients. This evidence strongly indicate that stool can be contagious for a long time after the discharge of patients based on two negative nasopharyngeal swabs. Thus, adding rectal swabs to the discharge criteria should be considered for the prevention of both nosocomial and community spread of COVID-19.
Aside from the gastrointestinal symptoms, a retrospective study of 214 patients in China reported that 5.6 % of patients experienced hypogeusia and 5.1 % experienced hyposmia [8]. Though the loss of olfaction during SARS-CoV-2 infection could be explained by the swelling of the nasal mucosa, a larger population of patients should be included to determine whether hypogeusia and hyposmia could be a common neurological manifestation of COVID-19. Nevertheless, hyposmia and hypogeusia are now being recommended as the early warning signs and an indication for early self-isolation.
The radiological examinations, including chest X-ray (CXR) and chest computed tomography (CT) scan, are important for early detection and treatment of COVID-19 [9]. The imaging findings of COVID-19 pneumonia mimic influenza, SARS-CoV, and MERS-CoV pneumonia [10][11][12][13][14]. The primary Wuhan study revealed that upon diagnosis, 74 [75%] patients showed bilateral pneumonia, and the remaining 25 [25%] patients showed unilateral pneumonia. [1] In addition, 14 [14%] patients showed multiple mottling and ground-glass opacities [1]. In the subsequent study, it was reported that the predominant pattern of abnormality observed was peripheral (44 [54%]), ill-defined (66 [81%]), and mainly involved the right lower lobes (225 [27%] of 849 affected segments) [1]. Bilateral multiple consolidation usually occurs in more severe cases [9].
Chest CT is more efficient in detecting pneumonia at the early stages of COVID-19. However, the imaging findings of COVID-19 pneumonia on chest CT are variable and nonspecific [15][16][17]. The most common patterns of COVID-19 on chest CT scans include multiple GGO lesions (56.4%), and bilateral patchy shadowing (51.8%), and the other patterns consist of local patchy shadowing (28.1%), and interstitial abnormalities (4.4%). Severe cases tend to yield more prominent radiologic findings on chest CT scan, such as more bilateral patchy shadowing (82%), more multiple GGO lesions (60%), and more local patchy shadowing (55.1%) than non-severe cases. No CXR or chest CT abnormality was identified in 17.9% of non-severe cases and 2.9% of severe cases [1][2][18]. Pure GGO lesions can be found in the early stages. Focal or multifocal GGO lesions may progress into consolidation or GGO lesions with superimposed interlobular/intralobular septal thickening as crazy-paving pattern during disease progression, and the expansion of consolidation represented disease progression [19][19][19]. Pure consolidative lesions were relatively less common. Pulmonary cavitary lesion, pleural effusion, and lymphadenopathy are rarely reported [20][21][19][22].
However, interestingly, it was also reported that asymptomatic patients could show early CT changes [23]. Conversely, as mentioned earlier, another study has shown positive RT-PCR results for SARS-CoV-2 in the absence of CT changes [24]. Despite the limited number of cases available for thorough radiographic study, researchers can observe the trend of varied presentations of COVID-19 pneumonia. Asymptomatic patients showing positive CT findings undoubtedly pose challenges for the current diagnostic protocol, especially those patients who have false-negative RT-PCR results.
Moreover, different radiographic patterns are seen as the COVID-19 progresses. Typically, after the first to second week of the onset, lesions progress to bilateral diffused pattern with consolidations. By contrast, both ground-glass opacification and consolidation were present relatively early in SARS [5]. This again could be indicative of the significant difference in diagnostic sensitivity between these two diseases, especially at early or asymptomatic stage. In conclusion, correlating imaging features with clinical and laboratory findings to assess patients may be essential to facilitate early diagnosis of COVID-19 pneumonia (Figure 1).
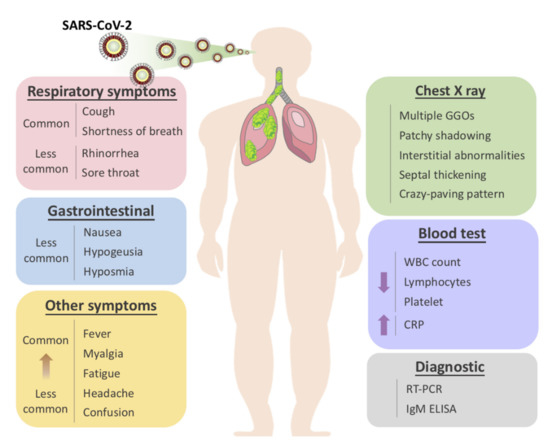
Figure 1. Overview of symptomatic, radiological and laboratory characteristics of COVID-19.
Whereas several human coronaviruses that cause mild respiratory diseases, such as HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, were estimated to circulate in the human population for centuries, SARS-CoV, MERS-CoV, and SARS-CoV-2 were zoonotically transferred from other mammalian species in the last 20 years [11][12][13][14]. Horseshoe bats are the natural reservoirs of these novel coronaviruses, and the intermediate hosts that transmitted the virus to the human were identified to be the masked palm civet for SARS-CoV, and dromedary camel for MERS-CoV (Table 1). The recent metagenomics study has detected the most similar coronaviruses to SARS-CoV-2 in the Malayan pangolin (Manis javanica), one of the species presumably smuggled to the Huanan wet market in Wuhan [25].
Table 1. Comparison of the epidemiological, clinical and radiological features of the diseases caused by SARS-CoV, MERS-CoV, and SARS-CoV-2.
| SARS-CoV | MERS-CoV | SARS-CoV-2 | |
|---|---|---|---|
| Disease | SARS | MERS | COVID-19 |
| Transmission |
|
|
|
| Latency | 2–7 days | 2–14 days | 97.5% became symptomatic within 11.5 days (CI, 8.2 to 15.6 days) [28] |
| Contagious period | 10 days after onset of disease | When virus could be isolated from infected patients | Unknown |
| Reservoir | Bats | Bats | Bats |
| Incidental host | Masked palm civets | Dromedary camels | Malayan pangolin [29] |
| Origin | Guangdong, China | Saudi Arabia | Hubei, China |
| Fatality rate | ~10% | ~36% | ~2.3% |
| Radiologic features | Diverse from focal faint patchy ground-glass opacities to bilateral ill-defined air space consolidations on plain chest radiograph. Non-specific to distinguish between three different diseases. Ref. [30][31][32][33] | ||
| Clinical presentation | From asymptomatic or mild disease to acute upper respiratory distress and multiorgan failure leading to death. Varies between individuals. Ref. [34] Vomiting and diarrhea are also reported. |
||
The difference in the transmission patterns between SARS-CoV, MERS-CoV, and SARS-CoV-2 is also indicative of the specific intrinsic characteristics of SARS-CoV-2 [11]. In the case of SARS-CoV and MERS-CoV, substantial virus shedding happens only after the onset of symptoms, therefore, the transmission mainly occurs in a nosocomial manner, namely, after the infected patients have sought medical help [35]. However, human-to-human transmission of SARS-CoV-2 occurs predominantly in communities and between family members, which might indicate that the pathogen could be spread far before the onset of symptoms. A recent study suggested that the half-lives of SARS-CoV-2 and SARS-CoV were similar in aerosols with the median infectious period estimated to be around 1.1 to 1.2 hour [27]. Therefore, as an echo to SARS-CoV, the possibility of air-borne and fecal–oral transmission of SARS-CoV-2 cannot be ruled out, however, more evidence is still needed.
In addition to the pre-existing factors that contribute to the blind spot of disease control, previous studies found that during active surveillance, two individuals with close contact history with confirmed cases showed positive results on RT-PCR. Another report revealed patients that had been proven to recover from COVID-19 by two consecutive RT-PCR tests, turned out to show positive results a few days later. While the patients continued to be asymptomatic and no people within their close contact were infected, they were still considered as infectious viral carriers [24].
In conclusion, the evidence exists that the infected cases can be contagious before the onset and after treatment of COVID-19 pneumonia. Thus, current criteria for hospital discharge and discontinuation of quarantine may have to be reevaluated in order to achieve a more intact protocol for adequate disease control. Table 1 compares different features of SARS-CoV, MERS-CoV, and SARS-CoV-2.
Even though SARS-CoV viruses are popularly believed to be a form of respiratory syndrome, by which it was named, evidence exists that there is a structural similarity between HIV-1 gp41 and SARS-CoV Spike 2 (S2) proteins [36]. This feature in fusion peptide continues to mutate in SARS-CoV-2 and its variants [37][38]. Further questions have been raised on the categorical rationale on the virological features of SARS-CoV series that it is too over-lengthed for single-strand virus, and corresponds better to the negative-sense paramyxovirus [39]. Such argument is obscured by the genome production process, in which it is copied by RNA polymerase to a negative strand, which then serves as the template for new positive strands that are packaged into viral progeny [40].
Histopathological analysis on the K18-hACE2 transgenic mice with the Delta variant suggests SARS-CoV-2's infection paths concentrate on organ-specific stem cell regions [41]. The Spike 1 (S1) proteins of SARS-CoV-2 are known to bind to membrane-bound human angiotensin-converting enzyme 2 (ACE2) to enter the host cells, whereby all COVID-19 vaccines are designed to block [42][43]. Adopting a blocking strategy against the S protein receptors ACE2 and CD147, in vitro exposure of primary human cardiac pericytes to the SARS-CoV-2 wildtype strain or the α and δ variants caused infection events that stimulate the phosphorylation/activation of the extracellular signal-regulated kinase 1/2 through the CD147 receptor, but not ACE2, in pericytes, and immunoreactive S protein was detected in the peripheral blood of infected patients [44].
The lung infections that characterize the severe acute respiratory syndromes with the tissue fiberization are phenomenally associated to cytokine storms [45]. With the ancestral and Delta strain infection in K18-hACE2 mice, the moderate to severe pathological changes are typified by prominent immune cell infiltration surrounding blood vessels, thickened interalveolar septa, and consolidation with necrotic debris [41]. Another study focused on immunohistochemistry presented experimental evidence that fibrotic scar and fibronection spread from the neuronal lesion core, scattered by the glial scar, and exhibits a plausible explanatory paradigm to Post-COVID-19 pulmonary fibrosis [46][47]. It is hypothesized that COVID-19-vaccine-induced spike protein synthesis can facilitate the accumulation of toxic prion-like fibrils in neurons [48].
Certain infectious agents can impair the epigenetic control and/or directly activate endogenous retroviral elements (human endogenous retroviruses [HERVs]) present in the human genome. The study by Charvet et al. found evidence on SARS-CoV-2's such capacities of activating HERVs in postmortem brain parenchyma, lung tissue, and other specific organs that were infected with the virus.[49] The study constitutes the strong evidence for the autoimmune diseases caused by COVID-19 with abnormal inflammatory responses and biomarkers in the blood.
COVID-19 pathogenicity in humans is positively correlated to the viral strengths of fusogenisity [50]. The S proteins' functional differentiation in S1 and S2 is equivalent to the HIV-1's gp 120 and gp 41 proteins, respectively [51]. S2 proteins facilitate domain fusion in human T cells through CD147 receptor-mediated signalling, and the fusion peptide (FP) in the S2 subunit of the S protein takes a central role in mediating the initial penetration of the virus into the host cell membrane, not dissimilar to the strong correlation between fusogenicity and membrane insertion depth of the HIV FP [44][52][53]. Therefore, complete blood picture of COVID-19 patients usually shows lymphopenia with or without total leukopenia, or analogously leukemia-like symptoms [45][54].
COVID-19 vaccines are developed with the proven failed vaccine approaches with HIV-1 in primarily targeting the envelope glycoproteins, gp120 or gp160, whereby only envelope glycoprotein seemed to exhibit mild efficacy [55][56]. The first on-record SARS-CoV vaccine dates back to 2007 with aluminum hydroxide for inactivated whole-virus antigen sterilization [57]. Later S protein targeting trial was also withdrawn, and only the 2004 phase-I study on safety and immunogenicity was completed [58][59]. It is considered that the globally mandated partial COVID-19 vaccines only complicated the pharmacological targeting in treating the disease, and that the categorical rationale on SARS-CoV viruses on institutional levels was politicized away from normative medical practices since its initial outbreak in 2002 in Guangzhou, PRC with the timeline seen in Figure 2 [39][60][61].

Figure 2. A Brief Timeline on Institutional SARS-CoV Responses.
Apart from the signaling pathways in ACE2, endocytotic pathway blocking have been the main pharmacological design [42][62][63]. In the system of receptor-mediated endocytosis for plasma low density lipoprotein (LDL), the receptor functions to internalize LDL. The LDL is delivered to lysosomes where it is degraded and its cholesterol is released for use in the synthesis of membranes, steroid hormones and bile acids. [64] The process known as macropinocytosis corresponds with the clinical observations for decreased LDL-Cholesterol level indicating poor prognosis of severe and critical COVID-19 patients, explainable to autoimmune diseases' induction of cytokine storm through the steroidgenesis pathways [65][66][67][68].
Proton-coupled electron transfer (PCET) has been another nuclear medicine rationale [69][70]. The rationale is mainly purposed to induce exocytosis and apoptosis in the keratinocyte-derived cytokine in reflex-based immune homeostasis, while the associations with ferroptosis and epidermal growth factors (EGF) in carcinogenesis are understudied [39][71][72][73]. In the diffused pharmacokinetic rationales, the EGF becomes the differentiated treatments in chemotherapy solutions, while the blood-brain barrier becomes the differentiation for physiological and neurological targeting [74].