1000/1000
Hot
Most Recent

Hepatocellular carcinoma is the most prevalent form of liver cancer in the world. Annually, HCC affects approximately 900,000 individuals, and over 70% of new cases are diagnosed in Asia. The etiology of HCC is complicated due to the multiple risk factors involved.
The etiology of HCC is complicated due to the multiple risk factors involved [1]. HCC usually arises from a background of chronic liver disease caused by alcohol abuse, metabolic syndrome, hepatitis B or C infections and/or aflatoxin exposure that eventually scars the liver parenchyma, leading to the irreversible condition of liver cirrhosis and the subsequent development of HCC [2]. Most HCC patients are diagnosed with advanced disease, and the majority of them are unresectable due to the early dissemination of cancer cells inside the liver. HCC tends to grow near blood vessels such as the portal vein or hepatic vein, which makes surgery an impossible task. α-Fetoprotein (AFP) is commonly used as a serum biomarker for early detection of HCC and also for evaluation of the prognosis and monitoring of response to therapy [3][4]. Systemic chemotherapy for many years is ineffective and therefore not an option for HCC, due to the highly resistant nature of HCC. Locoregional therapies including percutaneous ablation, transarterial chemoembolization (TACE) and radioembolization serve as the main alternative therapies, but largely depend on tumor location, burden and other complications [5]. Ablation is the first line option over surgery for unresectable HCC; however, treatment outcome is still disappointing and recurrence is often seen. In 2008, a tyrosine kinase inhibitor (TKIs), sorafenib, was the first approved targeted therapy to be used as a first-line drug to treat advanced HCC [6]. Subsequently, three additional TKIs, lenvatinib, regorafenib and cabozantinib, have been approved and become available for use in first-line and second-line settings and found to provide beneficial effects and prolonged survival [7][8]. Despite of the application of TKI therapy, median survival with advanced HCC remains unsatisfactory at less than two years [5][9]. Recently, ramucirumab, a therapeutic monoclonal antibody drug that acts against vascular endothelial growth factor (VEGF) receptor 2, has shown significant survival benefits in patients with increased AFP (>400 ng/mL) after sorafenib [10]. A VEGF-neutralizing antibody, bevacinumab, has also proven effective in a single-agent phase II trial in HCC patients. However, serious bleeding complications were observed in 11% [11].
Immune surveillance plays an important role in identifying and eliminating normal cells that become malignant. As when fighting invading pathogens, the innate and adaptive immune systems work together to form an anti-tumor army to destroy cancer cells. However, the interplay between cancer cells, stromal cells, and infiltrating immune cells eventually creates an immunosuppressive tumor microenvironment (TME) that leads to immune evasion, which was previously considered impossible to reverse. There are two main aspects explaining the formation of an immunosuppressive TME. First, as HCC progresses, cancer cells recruit immune cells such as myeloid-derived suppressor cells (MDSCs) or M2 tumor-associated macrophages (TAMs) and FoxP3 + regulatory T cells (T reg ), which are known to help the tumor grow better, by secreting chemokines, cytokines and growth factors to form a tumor-promoting niche. As a result, the accumulation of tumor-promoting immunes cells eventually comprises an area of anti-tumor immunity. Second, upregulation of co-inhibitory molecules such as immune checkpoint ligand PDL1 and increased expression of tolerance-related enzymes such as indoleamine 2,3-deoxygenase (IDO) and arginase-1 in cancer cells or tumor-infiltrating immune cells also contribute to the formation of immunosuppressive TME. In addition, downregulation of tumor-associated antigens (TAAs), also known as tumor antigen escape [12][13], and reduced recognition of TAAs by immune cells through alterations in the antigen-processing machinery both play a significant role in the promotion of tumor progression [14]. Therefore, immunotherapies aiming to reverse and overcome the immunosuppressive TME in order to effectively enhance the activity of tumor-killing immune cells point out the future direction of HCC therapy.
The liver is the organ responsible for the detoxification of gut-derived blood and systemic circulation. Therefore, the frequent exposure of liver cells to food antigens and to microbial products generated by gut bacteria shapes the dynamic complexities of a liver microenvironment that fosters immune tolerance [15]. This tolerogenic milieu is maintained by liver antigen-presenting cells (APCs), including resident kuffer cells (KCs), sinusoidal endothelial cells (SECs) and stellate cells (SCs), through the secretion of an array of immunosuppressive cytokines, chemokines and growth factors [16]. Kuffer cells are known to express immunosuppressive cytokine IL-10 and IDO, and prostaglandins to promote T reg activation [17][18]. Myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs) and regulatory T cells also produce IL-10 to attenuate the ability of APCs to stimulate T cells and to promote PDL1 expression in monocytes [19]. TGF-β is a well-known soluble factor that attenuates the anti-tumor response by inhibiting the activation of dendritic cells (DCs) [20] and polarizing macrophages towards the M2 phenotype [21], as well as inducing T reg cell activation [22]. In HCC TME, TGF-β is mainly produced by cancer cells, T reg cells and macrophages [15]. High serum TGF-β has been linked to poor prognosis of patients with HCC after sorafenib treatment [23]. SCs-derived hepatocyte growth factor (HGF) also promotes the infiltration and accumulation of MDSCs and T reg cells inside the TME [24][25]. Vascular endothelial growth factor (VEGF) produced by cancer cells or MDSCs plays an essential role in promoting angiogenesis, leading to the formation of an abnormal tumor vasculature that not only serves as a barrier for cytotoxic T lymphocytes (CTLs) but disables them by expressing PDL1 and Fas ligand [26][27][28]. Aside from its role in inducing abnormal tumor vasculature, Courau, et al. showed that VEGF and TGF-β could cooperatively foster immunotolerant TME by blunting the antigen-presenting functions of DC and generating MDSCs [29].
Co-inhibitory molecules expressed by the effector lymphocytes fall into a category of immune checkpoint which serves the purpose of preventing overactivation of lymphocytes upon the engagement of APCs like DCs or macrophages [30]. To evade immune surveillance, tumor cells exploit this mechanism to express the corresponding ligands of co-inhibitory molecules to blunt effector T cells or macrophages. Program death 1 (PD1), cytotoxic T lymphocyte-associated antigen 4 (CTLA4) , T cell immunoglobulin and mucin domain containing-3 (TIM3), lymphocyte-activation gene 3 (LAG3), and siglec-10 are among the most extensively studied immune checkpoints [31][32]. PD1 has been found to be expressed by activated T cells, nature killer (NK) cells, MDSCs, monocytes and DCs [33][34][35]. Its corresponding ligand, PDL1, has been found in tumor cells, stromal cells, and myeloid lineage cells like macrophages and DCs [35]. CTLA4 is predominantly expressed by T reg cells and only upregulated in activated T cells [36][37]. In addition, the sialic acid-binding immunoglobulin (Ig)-like lectins 10 (siglec-10) has recently been identified as a new class of co-inhibitory molecule expressed by macrophages to interact with its corresponding ligand, CD24, expressed on the tumor surface [32][38].
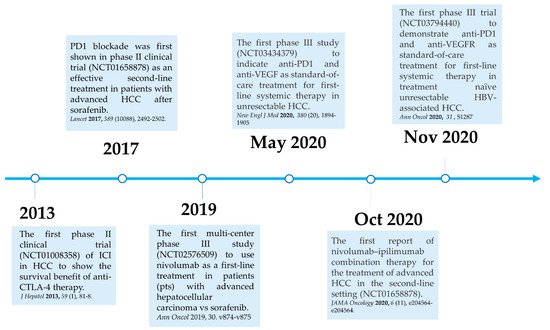
Here, we summarize the current milestones regarding recent clinical trials testing ICIs as a potential systemic treatment for advanced HCC ( Figure 1 ). The first immune checkpoint inhibitor (ICI), ipilimumab, the anti-CTLA-4 monoclonal antibody (mAb), was approved by the U.S. Food and Drug Administration (FDA) in March 2011 for the treatment of patients with advanced melanoma [39]. In 2013, a pilot clinical trial involving 20 patients with advanced HCC and a background of chronic hepatitis C virus (HCV) infection who received tremelimumab treatment, another anti-CTLA-4 mAb, showed promising results in terms of safety, antitumor and antiviral activity [40]. This encouraging result has led to the approval of another ICI, the PD1 inhibitor nivolumab, for the treatment of patients after sorafenib failure in the CheckMate 040 phase I/II clinical trial. In the CheckMate 040 trial ( n = 262), durable objective response rate (ORR), defined as the sum of complete (CR) and partial (PR) response rates, was observed in 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase, The median overall survival (OS) in sorafenib-experienced patients was 16.5 months. Further, the two-year survival rate among the responders was over 80% [41]. Soon after, nivolumab was approved by the FDA as a second-line systemic therapy for advanced HCC after sorafenib. Another PD1 inhibitor, pembrolizumab. was also shown effective and tolerable in patients with advanced HCC after sorafenib in a non-randomized, open-label phase II trial. ORR was recorded in 17% 95% CI 11–26) of patients previously treated with sorafenib [42]. Given the consistent results in terms of anti-tumor activity and safety from different PD1 mAb, a randomized, double-blind, phase III trial (KEYNOTE 240) ( n = 413) testing pembrolizumab versus placebo after sorafenib failure was launched in a second-line setting. Median OS was 13.9 months (95% CI 11.6–16) in the pembrolizumab treatment group and 10.36 months (95% CI 8.3–13.5) in the placebo group, and a statistically significant survival benefit was observed (Hazard ration HR 0.78; p = 0.0238) in the final analysis [43]. Although, OS and progression-free survival (PFS) did not reach the prespecified criteria, the results were in line with KEYNOTE 224, indicating a favorable risk-to-benefit ratio in the pembrolizumab group. Table 1 summarizes current important phase III trials involving ICI therapy as a major treatment modality.
| Trial Identifier | ICI/Isotype | Drug | Treatment Arm | Eligible Patient/Setting | Endpoint | Ref. |
|---|---|---|---|---|---|---|
| Monotherapies | ||||||
| NCT02702401 | PD1/IgG4 | Pembrolizumab | 1. Placebo 2. Pembrolizumab |
Advanced HCC/2L | Dec. 2022 | [43] |
| NCT02576509 | PD1/IgG4 | Nivolumab Sorafenib |
1. Sorafenib 2. Nivolumab |
Advanced HCC/1L | June 2021 | [44] |
| NCT03755739 | PD1/IgG4 | Pembrolizumab | 1. Peripheral in fusion | Advanced HCC/1L | Nov. 2021 | Ongoing |
| 2. Artery infusion | ||||||
| 3. Intra-tumor infusion | ||||||
| NCT03062358 | PD1/IgG4 | Pembrolizumab | 1. Placebo 2. Pembrolizumab |
Advanced HCC/2L | Jan. 2022 | Ongoing |
| NCT03412773 | PD1/IgG4 | Tislelizumab | 1. Placebo 2. Tislelizumab |
Unresectable HCC/1L | May 2022 | [45] |
| Combination therapies of ICI and TKI or anti-angiogenic agents | ||||||
| NCT03298451 | PD1 CTLA-4 (IgG4) |
Durvalumab Tremelimumab |
1. Sorafenib 2. Tremelimumab 3. Tremelimumab plus durvalumab |
HCC BCLC stage B not eligible for locoregional therapy/1L | June 2021 | [46] |
| NCT03794440 | PD1/IgG4 VEGF/IgG1 |
Sintilimab Bevacizumab biosimilar |
1. Sorafenib 2. Sintilimab plus Bevacizumab biosimilar |
Advanced HCC/1L | Dec. 2022 | [47] |
| NCT03847428 | PDL1/IgG1 VEGF/IgG1 |
Durvalumab Bevacizumab |
1. Combination with resection/MWA 2. Resection/MWA alone |
HCC eligible for curative resection/MWA/2L | June 2023 | [48] |
| NCT03713593 | PD1/IgG4 VEGFR |
Pembrolizumab Lenvatinib | 1. Lenvatinib 2. Pembrolizumab |
Advanced HCC/1L | July 2022 | [49] |
| NCT03764293 | PD1/IgG4 TKI |
Camrelizumab Apatinib |
1. Apatinib | Advanced HCC/1L | Jan. 2022 | Ongoing |
| NCT03434379 | PDL1/IgG1 VEGF/IgG1 |
Atezolizumab Bevacizumab |
1. Sorafenib 2. Atezolizumab plus Bevacizumab |
Advanced HCC/1L | June 2022 | [50] |
Aside from PD1/PDL1 and CTLA-4, here we discuss other important co-inhibitory molecules expressed by T cells which have been identified as potential immune checkpoints that modulate T cell activation. T-cell immunoglobulin and mucin-domain containing-3 (TIM3) is a membrane-bound protein that is originally expressed in CD8 + cytotoxic T cells and interferon-γ-producing CD4 + T helper 1 (Th1) cells [51]. Initially, the function of TIM3 was associated with autoimmune disease. Blocking of TIM3 with TIM3-specific antibody or administration of TIM-3 immunoglobulin (Ig) fusion protein resulted in Th1 cell and macrophage hyperactivation in an experimental autoimmune encephalomyelitis (EAE) mouse model [52]. Later, TIM3 expression was found in several immune cells, including T reg cells [53] , myeloid cells [54], natural killer (NK) cells [55] and dendritic cells (DCs) [51]. Notably, co-expression of TIM3 and PD1 has been observed in dysfunctional Th1 cells that express low levels of IL-2 and IFN-γ in the preclinical model [56]. A synergistic anti-tumor effect of combined anti-PD1 and anti-TIM3 immunotherapy has been observed in solid tumors in mouse models [56][57]. Therefore, combination treatment of TIM3 and PD1 or CTLA-4 inhibitor could serve as an attractive ICI regimen for HCC. Recently, a phase II trial assessing the efficacy and safety of anti-PD1 and anti-TIM3 combination therapy (NCT03680508) has been launched; the results are still being awaited.
Another important aspect to examine is that compensatory upregulation of TIM3 and LAG3 may confer resistance to anti-PD1/PDL1 treatment. In a preclinical mouse model, Oweida et al. demonstrated that TIM3 upregulation was observed in tumor-infiltrating CD4 and CD8 T cells in murine head and neck squamous cell carcinoma (HNSCC) tumors treated with radiotherapy (RT) and anti-PDL1 therapy. Combined treatment with anti-TIM3, anti-PDL1 and RT led to the promotion of T cell cytotoxicity, decreased T reg and significant tumor regression as compared with anti-PDL1 plus RT treatment [58]. In an effort to address the resistance mechanisms of PD1 blockade, Limagne et al. showed that accumulation of galetin-9-expressing monocytic MDSCs (mMDSCs) and TIM3-expressing CD8 T cells was found in lung cancer patients with resistance to anti-PD1 therapy, and may play a crucial role in resistance to PD1 blockade. They demonstrated that anti-TIM3 antibody in vitro could reverse resistance to anti-PD1 in peripheral blood mononuclear cells (PBMCs) isolated from lung cancer patients. Moreover, galetin-9-expressing mMDSCs could impede TIM3 + CD8 T cell activity to reduce anti-PD1 treatment efficacy [59]. In addition, Jikova et al. analyzed fresh HCC biopsies and peripheral blood samples from 21 HCC patients treated with sorafenib or PD1/PDL1 blockade therapy to show that non-responders tended to have TIM3 and LAG3 upregulation on circulating T cells compared with responders [60].