1000/1000
Hot
Most Recent

Urinary tract infections (UTIs) belong among the most common bacterial infections. They comprise the contamination of the periurethral space by specific uropathogens residing in the gut, followed by urethral colonization and pathogen ascension to the urinary bladder. Studying the association between gut microbiota and subsequent development of bacteriuria and UTI is of great interest and importance. Nevertheless, with discovering a multifaceted, symbiotic microbiome in the healthy urogenital tract, the well-established diagnostic and therapeutic approaches for the urinary tract infections (UTIs) need to be re-assessed. Precisely, emerging data suggest that vaginal dysbiosis may result in Escherichia coli colonization and prompt recurrent UTIs. At the same time, urinary microbiome perturbations may precede UTIs' development and other pathologic conditions of the urinary system. Therefore, by the thoroughly assessment of specific gut, urinary tract, and genital tract microbiomes regarding their potential influence on UTI development, knowledge for the incidence reduction and new treatment approaches will be obtained.
During evolution, microorganisms developed intimate relationships with humans by colonizing various body environments at the interface with the outer part of the body and invaginations such as the skin, nose, mouth, gut, vagina, and urogenital tract—constituting, in turn, an integrated metaorganism [1][2]. The result is a reciprocal adaptation and functional consolidation, which confers substantial advantages to humans and their colonizers (primarily bacteria). Furthermore, our immune system has co-evolved with the resident microbiota and given rise to complex mechanisms for recognizing and destroying invading microorganisms while preserving its bacterial species [2][3]. There have been numerous studies over the last decades exploring the role of gut microbiota in health and disease, followed by studies of other high-volume microbiome organs such as the vagina and the skin. Though, fewer reports exist on the role of the microbiota at different body sites such as the urinary tract [4].
Urinary tract infections (UTIs) belong among the most common bacterial infections, affecting approximately 150 million people globally every year [5]. With incidence increasing with age and a lifetime incidence of 50–60% in adult women [6], UTIs are a significant burden on society and the healthcare system, not in the least due to treatments that contribute significantly to antibiotic resistance [7][8]. A wide range of pathogens causes these infections, and the underlying pathogenesis is usually explained by the ascending of intestinal bacteria. However, recent studies have implied the significant roles of the vaginal, intestinal, and urinary microbiota in disease activity regulation.
The origin of most bacterial UTIs is presumed to be in the gut [3], hence rendering it a natural first step in the investigation of the relationship between intestinal microbiota and subsequent development of bacteriuria and UTI. The well-established diagnostic and therapeutic paradigm for UTIs has recently come into question with the revelation of a multifaceted, symbiotic microbiome in the healthy urogenital tract [9][10]. In women, vaginal bacteria play a pivotal role in the pathogenesis of UTIs. Simultaneously, the gut microbiota is the ultimate source of bacterial strains responsible for cystitis and pyelonephritis in most cases, clearly demonstrating the crosstalk and interconnectedness of these two niches [11] (Figure 1). Therefore, recognizing factors that affect both gut and vaginal microbiota is indispensable for understanding UTI's pathogenesis and designing interventions to prevent it.
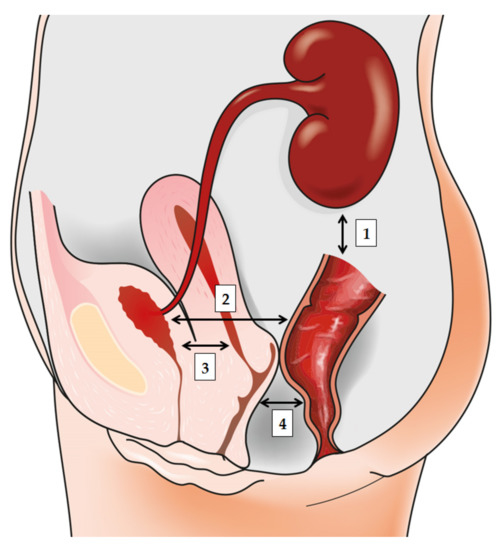
Figure 1. The bidirectionally informed communication between gut, urinary tract, and genital tract: (1) the gut-kidney axis plays a role in various renal disorders; (2) the gut-bladder axis and the link between ‘intestinal bloom of uropathogens’ and UTI development; (3) the gut-vagina axis where intestinal dysbiosis may influence local vaginal milieu; and finally, (4) the vagina-bladder axis where vaginal dysbiosis may prompt UTI development to act as a reservoir for Escherichia coli or prompt “covert pathogenesis”.
With the introduction of affordable sequencing techniques, metagenomic approaches independent of culture have shined a more detailed light on urinary microbiota's bacterial diversity. Since urine has been traditionally seen as naturally sterile [12][13] due to a plethora of methodological biases, novel techniques have moved away from the consideration of only dominant bacteria from rapidly and aerobically cultured urinary specimens [14]. Likewise, the higher frequency of UTIs in women than in men has prompted considerations that the source of bladder colonization is genital due to the small size of the female urethra [15]. Based on this, the well-known hypothesis states that the bladder microbiota is of vaginal origin (apart from UTIs), overlooking the fact that men also have UTIs and urinary microbiota [16]. The question remains on the way the UTIs link to the gut microbiota instead of vaginal ones.
Finally, there is no consensus on a strict definition of a UTI [17]. The bacterial colony-forming unit-based thresholds that delineate infection by standard clinical urine culture are still a matter of debate. The discovery further complicates this ambiguity that urinary bacteria and, thus, “bacteriuria” can be found in the urine of almost every individual—including those that do not present with urinary symptoms [18] and the effect has to therefore be refined beyond the mere presence/absence of particular taxa. As asymptomatic bacteriuria has even been viewed as a protective parameter against recurrent UTI [19], our improved understanding of the urinary microbiome may suggest mechanisms for this clinical observation and inform further translational events.
It is well-known that the composition of gut microbiota can influence distant body organs [20]. The majority of studies in the past decade have focused on characterizing the complex interactions in the microbiota gut-brain axis, which are crucial for maintaining human psychological and physiological wellbeing and associated with various neuropsychiatric disorders [21][22][23][24]. Like the gut and the brain, a bidirectional relationship also exists between the gut and the kidney. Mounting evidence indicates that gut microbiota plays an essential role in the gut–kidney axis [25] with the dysbiosis of the gut microbial community implicated in the pathogenesis of various renal disorders, thus indirectly contributing to hypertension and chronic kidney disease [26], as well as urinary stone disease [27] (Figure 1). A more direct link between gut microbiota dysbiosis and the urinary system is evident in urinary tract infections.
The UTI pathogenesis typically starts with contamination of the periurethral space by uropathogens residing in the gut, followed by colonization of the urethra and ascending migration to the bladder [28]. UTIs are predominantly caused by uropathogenic Escherichia coli (UPEC), which is responsible for over 80% of community-acquired infections, while healthcare-related infections are associated with Staphylococcus, Klebsiella, Enterobacter, Proteus, and Enterococcus [28][29]. UPEC strains are found in abundance in the gut of patients with UTIs and are thus considered to originate from the gut [30][31]. UPEC differs from commensal E. coli by possessing extragenetic material encoding for genes involved in bacterial pathogenicity, such as adhesins, toxins, surface polysaccharides, flagella, and iron-acquisition factors [32][33]. UTIs are more prevalent in women; the female urethra is closer to the anus and shorter than the male urethra, facilitating the migration to and colonization of gut microorganisms in the urinary tract [15][34].
According to the research reports, breastfeeding exhibits a protective effect against UTI in infants and preterm neonates, supporting the hypothesis that the origin of UTI is in the gut [35][36]. Furthermore, Paalanne et al. noted several differences at the family and genus levels, most notably the higher abundance of Enterobacter in the gut microbiota of paediatric patients with UTIs when compared to healthy controls, suggesting the intestinal environment and its microbial community is associated with the risk of UTI in children [37].
A recent study from Magruder et al. further described the gut microbiota–UTI axis [38]. The authors demonstrated that the increased abundance of E. coli in the gut was associated with the future development of E. coli bacteriuria and E. coli-induced UTI. Also, the E. coli strains in the gut displayed a more pronounced resemblance to the E. coli strain in the urine from the same subject, thus supporting the hypothesis that gut microbiota is a urinary tract colonization source [38].
Likewise, by combining semiquantitative culturing with comparative genomics, Thänert et al. provided evidence for the repeated transmission of uropathogens between the gut reservoir and the urinary tract, showing that recurrent UTIs (rUTIs) are habitually preceded by a so-called “intestinal bloom of uropathogens” [39]. The provided data expand our knowledge of the temporal dynamics of pathogen clearance and persistence following symptomatic UTI and underline the significance of acknowledging intestinal colonization with antimicrobial-resistant microorganisms as inherent to the pathophysiology of rUTIs [39].
Finally, a study by Magruder et al. from 2020 demonstrated that high relative abundances of two bacterial taxa—Faecalibacterium and Romboutsia—can be linked to the decreased risk of Enterobacteriaceae bacteriuria and UTI in kidney transplant recipients [40]. This research group additionally reported an inverse relationship of the relative abundances of the aforementioned two taxa with the relative abundance of Enterobacteriaceae, lending further support for a growing notion that intestinal commensal organisms are related with a lower risk of infectious complications, which is already deep-rooted for Clostridioides difficile infections [40].
Although antibiotics remain the commonly recommended treatment for UTIs, the long-term alteration of normal intestinal microbiota and emergence of multidrug-resistant microorganisms has made the advancement of alternative therapeutic options for combating the infection a necessity [41]. Along with the efforts in developing precision antimicrobial therapeutics and UTI vaccines, gut microbiota dysbiosis was recognized as one of the key contributing factors to developing UTIs, and modulating the gut microbial community could prove a promising strategy in disease prevention and treatment. The use of commensal bacteria as probiotics was demonstrated to reduce the abundance of pathogens, restoring the microbiota homeostasis [42]. Koradia et al. reported that the administration of a commercially available probiotic product containing two strains of lactobacilli supplemented with cranberry extract significantly lowered the number of recurrent UTIs in premenopausal women when compared to a placebo in a randomized, double-blind, placebo-controlled pilot study [43]. A similar study demonstrated a probiotic mixture was more effective than the placebo at reducing the risk of recurrent UTIs in children after their first episode of febrile UTI [44]. A noninferiority randomized controlled trial in postmenopausal women with recurrent UTIs confirmed oral probiotics (Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14) did not meet the noninferiority criteria in the prevention of UTIs when compared to antibiotic treatment [45]. Unlike antibiotic treatment however, the use of lactobacilli did not increase antibiotic resistance [45].
Recently, a very interesting link between fecal microbiota transplantation (FMT) and recurrent UTI was found, further confirming the gut microbiota–UTI axis and revealing a new potential treatment option for the disease. Tariq et al. reported a reduced UTI frequency after FMT for the treatment of recurrent Clostridioides difficile infection (rCDI) [46]. The study noted an improved antimicrobial susceptibility profile in microbial isolates from urine samples after FMT, suggesting gut decolonization of multidrug-resistant organisms in favor of less pathogenic and commensal microbes [46]. Several case reports confirmed beneficial effects of FMT for patients with rCDI or IBS who concomitantly suffered from rUTIs, demonstrating reduced or no UTI recurrences after the FMT procedure [47][48]. Moreover, two recent studies provided insight on the application of FMT for the treatment of rUTI in kidney-transplant recipient patients without concomitant disease [49][50]. Both author groups report a marked decrease or absence of UTI-inducing bacterial strains in the urine samples after FMT, and no symptoms of subsequent UTIs in a 12-month follow-up period, suggesting successful modification of the urinary microbiota by FMT and an important role of the gut microbiota composition in the pathogenesis of rUTIs [49][50]. A recent case report along those lines showed that the disappearance of UTIs in a patient presenting with recurrent infections of the urinary tract resulted from the reduction of Enterobacteriaceae in the gut microbiota (from 74% to 0.07%) after FMT [51]. A clinical trial determining the effectiveness of fecal transplantation in the treatment of refractory, recurrent urinary tract infections (NCT03050515) is currently underway, assessing change in frequency of UTIs following FMT as well as the efficacy of FMT in modifying the UTI bacterial profile in urine samples to commensal, pan-sensitive organisms.