1000/1000
Hot
Most Recent

Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly evolved into a global pandemic. The hyperglycemia in patients with diabetes mellitus (DM) substantially compromises their innate immune system. SARS-CoV-2 uses human angiotensin-converting enzyme 2 (ACE2) receptors to enter the affected cell. Uncontrolled hyperglycemia-induced glycosylation of ACE2 and the S protein of SARS-CoV-2 could facilitate the binding of S protein to ACE2, enabling viral entry. Downregulation of ACE2 activity secondary to SARS-CoV-2 infection, with consequent accumulation of angiotensin II and metabolites, eventually leads to poor outcomes. The altered binding of ACE2 with SARS-CoV-2 and the compromised innate immunity of patients with DM increase their susceptibility to COVID-19; COVID-19 induces pancreatic β-cell injury and poor glycemic control, which further compromises the immune response and aggravates hyperglycemia and COVID-19 progression, forming a vicious cycle. Sequential cleavage of viral S protein by furin and transmembrane serine protease 2 (TMPRSS2) triggers viral entry to release the viral genome into the target cell. Hence, TMPRSS2 and furin are possible drug targets. As type 1 DM exhibits a Th1-driven autoimmune process, the relatively lower mortality of COVID-19 in type 1 DM compared to type 2 DM might be attributed to an imbalance between Th1 and Th2 immunity. The anti-inflammatory effects of dipeptidyl peptidase-4 inhibitor may benefit patients with DM and COVID-19. The potential protective effects of sodium–glucose cotransporter-2 inhibitor (SGLT2i), including reduction in lactate level, prevention of lowering of cytosolic pH and reduction in pro-inflammatory cytokine levels may justify the provision of SGLT2i to patients with DM and mild or asymptomatic COVID-19. For patients with DM and COVID-19 who require hospitalization, insulin-based treatment is recommended with cessation of metformin and SGLT2i. Further evidence from randomized or case–control clinical trials is necessary to elucidate the effectiveness and pitfalls of different types of medication for DM.
Coronavirus disease 2019 (COVID-19), an infection caused by an enveloped virus with a single-stranded RNA genome, namely novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a global pandemic. Several physical conditions, including older age, severe obesity, and diabetes mellitus (DM) are associated with an increased risk of morbidity and death from COVID-19 [1]. Individuals with DM have a higher risk of nosocomial bacteremia, pulmonary infection and infectious disease because of reduced immune response. The impairments of the innate and humoral immune systems secondary to metabolic dysfunction in patients with DM render them susceptible to infectious diseases [2]. As patients with DM were associated with a poor prognosis of COVID-19 and COVID-19 tends to worsen the dysglycemia of these patients, complex interactions between DM and COVID-19 were proposed [3]. Researchers have sought to elucidate the association between COVID-19 and DM in order to improve the understanding of the potential mechanism and provide effective treatment for patients with DM and COVID-19. In this review, we discuss the possible pathophysiology and mechanisms, clinical characteristics and potential concerns regarding treatment choices for patients with DM and COVID-19.
Patients with DM have a risk of developing more severe COVID-19 [4]. In addition, patients with COVID-19 experience abnormalities of glucose metabolism, such as hyperglycemia, euglycemic ketosis and even classic diabetic ketoacidosis [5]. There are complicated crosslinks between hyperglycemia/DM and COVID-19 (Figure 1). Patients with DM have an increased risk of requiring critical care in the intensive care unit, requiring invasive mechanical ventilation, or mortality after infection with SARS-CoV-2 [6]. Hyperglycemia in these patients can significantly compromise their innate immune responses to infection [7]. Among patients with COVID-19, compared with those without DM, those with DM had higher levels of inflammation markers, including interleukin-6, C-reactive protein and ferritin, which suggested that the pro-inflammatory state of patients with DM was prone to rapid deterioration and subsequently poor COVID-19 outcomes [8].
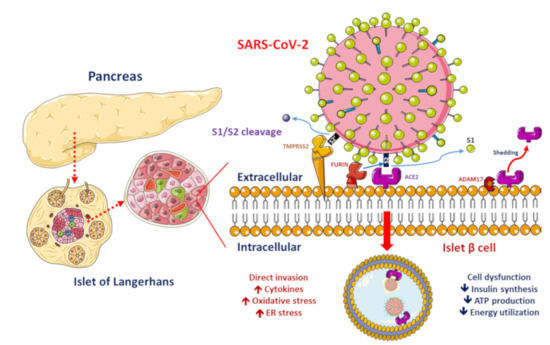
Figure 1. Proposed functions of pancreatic β-cell molecules for interaction with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
ACE2, ADAM17 and TMPRSS2/FURIN are present in the β-cell membrane in type 2 diabetic human pancreatic islets. The SARS-CoV-2 spike protein should be cleaved at two sites, namely S1/S2 and S2′, to trigger the fusion of viral and cellular membranes during virus entry to release the virus genome into the host cell. Initially, cleavage at the S1/S2 site by TMPRSS2 occurs, followed by cleavage at the S2′ site by FURIN. SARS-CoV-2 replication could be inhibited by the synthetic FURIN inhibitor. The direct binding of SARS-CoV-2 to ACE2 on β-cells might contribute to β-cell damage and insulin deficiency, which, combined with cytokine-induced insulin resistance and hypokalemia-related inhibition of insulin secretion, contributes to worsening glucose control in patients with diabetes mellitus.
SARS-CoV-2 uses the human angiotensin-converting enzyme 2 (ACE2) receptors to enter the affected cell [9]. ACE2 exists in the human body in both membrane-bound and soluble forms and viral entry of SARS-CoV-2 into host cells relies on binding of the viral spike (S) proteins to the membrane of ACE2 [10]. A previous study has reported increased activity of ACE2 in diabetic mice [11]. A phenome-wide Mendelian randomization study reported a causal link between DM and the increased expression of ACE2 [12]. Increased ACE2 levels were also found in patients with cardiovascular disease or those taking renin–angiotensin system (RAS) blockers [13]. A marked increase in mortality among DM patient with COVID-19 was demonstrated in a recent meta-analysis [14]. On the other hand, obesity and metabolic disorders play an important role in COVID-19 prognosis since the larger amount of ACE2 receptors in adipocytes serve as a reservoir for the SARS-CoV-2 virus [15][16]. However, it is still unclear whether solely elevated ACE2 levels pose a higher risk of SARS-CoV-2 infection. The downregulation of ACE2 activity secondary to SARS-CoV-2 infection with consequent accumulation of angiotensin II and metabolites might eventually lead to acute respiratory distress syndrome or fulminant myocarditis [13]. ACE2 converts angiotensin II to angiotensin 1–7 (Ang 1–7), which promotes vasodilation, and enhanced ACE2 activity has been shown to mediate a protective effect in patients with COVID-19 based on anti-inflammatory benefits due to the enhancement of ACE2 [17]. Therefore, the amount of glycosylated ACE2 receptor rather than total ACE2 receptor in patients with DM is likely related to the augmented viral entry [18]. Previous studies have shown a strong link between hyperglycemia and the severity of COVID-19 [19] and severe acute respiratory syndrome (SARS) [20]. A recent study demonstrated that glycosylation of the ACE2 receptor in humans substantially contributes to the binding of the SARS-CoV-2 (Figure 2) [21]. Structure–function studies have also shown that the S protein of SARS-CoV-2 is highly glycosylated [22]. Uncontrolled hyperglycemia might induce potential modifications in glycosylation of ACE2 and the S protein of SARS-CoV-2, which possibly enables the S protein to bind to ACE2 and alters the immune response to the virus [18]. A recent study proposed that the worse outcomes in patients with DM who develop COVID-19 could be related to the nonenzymatic glycation of ACE2 [23]. Therefore, patients with high blood glucose or DM are at a risk of developing more severe COVID-19, which might be attributed to the hyperglycemia related aberrant glycosylation or hyperglycemia-enhanced nonenzymatic glycation of ACE2 and SARS-CoV-2 spike protein.
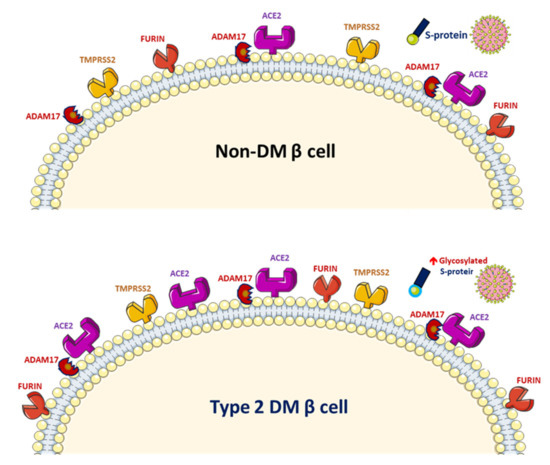
Figure 2. Increased glycosylated ACE2, ADAM17 and TMPRSS2 expression in human pancreatic islets and glycated SARS-CoV-2 spike protein in patients with diabetes mellitus (DM).
Both RAS and ACE2 play important roles in DM patients with COVID-19, especially through bradykinin being degraded by ACE and angiotensin 1–9 being activated by ACE2. Further, an atypical pattern of the RAS in COVID-19 patients was noted with a decreased ACE/ACE2 ratio and increased renin, angiotensin, kininogen, bradykinin and bradykinin receptors, which altogether induced hypotension, vasodilation, etc. and most of the symptoms related to patients with COVID-19 [24].
Worsening insulin resistance and glycemic control have been observed in patients with DM who develop SARS-CoV-2 infection, likely owing to the pro-inflammatory state induced by COVID-19 [3]. The elevated cytokine levels secondary to SARS-CoV-2 infection may cause impairments in pancreatic β-cell function and apoptosis [25]. In addition, ACE2 expression in the pancreas has been reported [26]. SARS-CoV was detected in the pancreas of patients whose death was attributed to SARS [27]. These findings suggest that direct binding of SARS-CoV-2 to ACE2 on pancreatic β-cells might contribute to their damage (Figure 1) and subsequent insulin deficiency and hyperglycemia, as observed previously in patients with SARS-CoV infection [28]. Available evidence shows that the direct β-cell damage [28], inflammation-induced insulin resistance [29], hypokalemia-related inhibition of insulin secretion by β-cells [30] and corticosteroid use in the treatment of COVID-19 contribute to the poor glycemic control in patients with DM who develop COVID-19 [3]. A previous study reported that patients with DM who were on insulin therapy required higher doses of insulin and those who were on oral antidiabetics required insulin therapy after admission owing to COVID-19 [8]. Hence, a vicious cycle secondary to SARS-CoV-2 infection in patients with DM was proposed, in that the altered ACE2 binding of SARS-CoV-2 and compromised innate immunity of patients with DM increase their susceptibility to COVID-19 and COVID-19 induces pancreatic β-cell injury leading to a worse glycemic profile, which, in turn, impairs the immune response, induces a proinflammatory state and eventually aggravates COVID-19 progression and poor glycemic control.
Several molecular mechanisms regarding the correlations between glucose metabolism and SARS-CoV-2 replication have been proposed. After viral entry of SARS-CoV-2 into human cells, glucose availability affects viral replication [31] and the utilization of excess glucose facilitates viral replication through the hexosamine biosynthetic pathway (HBP), which again induces overexpression of interferon regulatory factor–5 (IRF5), leading to subsequent endoplasmic reticulum stress, overproduction of cytokines, hyperinflammation and eventually multiorgan failure [32]. Among patients infected with influenza A virus, higher proinflammatory cytokines significantly correlated with increased sugar levels due to the essential function of HBP [33]. Evident findings revealed that IRF5 expression could be induced by persistent hyperglycemia, repetitive intermittent hyperglycemia and glucose fluctuations [34] and the contribution of daily postprandial glucose peaks after meals to the SARS-CoV-2 replication was proposed. These molecular mechanisms might provide hints for potential therapeutic strategies.
Patients with DM who develop COVID-19 should be divided into those with mild or asymptomatic COVID-19 and those with more severe COVID-19 who show symptoms or signs of decreased appetite, dehydration, hypoxia, sepsis and impaired renal function during either inpatient or outpatient treatment. For patients with mild or asymptomatic COVID-19, single or combination therapy with metformin, SGLT2i, and DDP4i should be considered to achieve favorable glycemic control and to take advantage of the other possible beneficial effects of these drugs. Clinicians should be cautious about the use of metformin and SGLT2i in patients with DM who have more severe COVID-19; intensive monitoring of glucose, fluid status, and renal function of these patients is warranted. Insulin-based treatment is recommended and cessation of metformin and SGLT2i should be considered for patients with DM and COVID-19 who require hospitalization to avoid the potential adverse effects of these drugs. As limited information is available regarding the outcome of patients with DM who develop COVID-19 and receive a specific category of medication for DM, further evidence from randomized or case–control (clinical) trials is necessary to elucidate the effectiveness and pitfalls of the different types of medication for DM.