The demand for pipeline steels has increased in the last several decades since they were able to provide an immune and economical way to carry oil and natural gas over long distances. There are two important damage modes in pipeline steels including stress corrosion cracking (SCC) and hydrogen induced cracking (HIC). The SCC cracks are those cracks which are induced due to the combined effects of a corrosive environment and sustained tensile stress. The present review article is an attempt to highlight important factors affecting the SCC in pipeline steels. Based on a literature survey, it is concluded that many factors, such as microstructure of steel, residual stresses, chemical Composition of steel, applied load, alternating current (AC) current and texture, and grain boundary character affect the SCC crack initiation and propagation in pipeline steels. It is also found that crystallographic texture plays a key role in crack propagation. Grain boundaries associated with {111}//rolling plane, {110}//rolling plane, coincidence site lattice boundaries and low angle grain boundaries are recognized as crack resistant paths while grains with high angle grain boundaries provide easy path for the SCC intergranular crack propagation. Finally, the SCC resistance in pipeline steels is improved by modifying the microstructure of steel or controlling the texture and grain boundary character.
- stress corrosion cracking
- residual stress
- AC current density
- crystallographic texture
- intergranular and transgranular cracks
1. Introduction
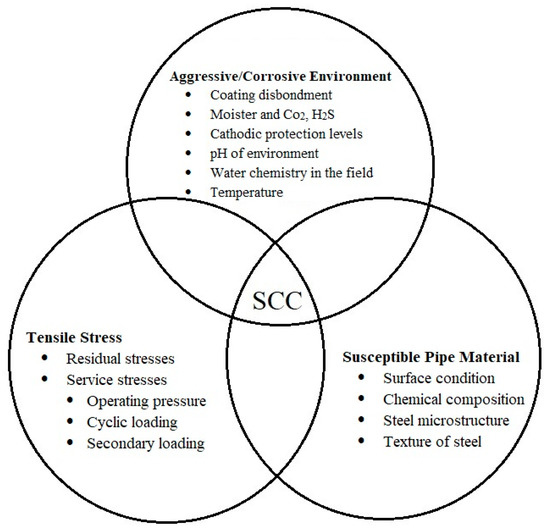
The demand for energy has increased in recent decades which forced the industry to develop high resistance pipeline steels [1,2,3]. Such steels show better mechanical properties and a higher corrosion resistance compared with normal carbon steels. However, these steels still suffer from two important failure modes including hydrogen induced cracking (HIC) and stress corrosion cracking (SCC) [4,5,6]. There are numerous studies in the literature focused on these failure modes. The SCC has been recognized as one of the main important failure modes in humid environments and causes a huge amount of economical loss and environmental disasters all around the world. The SCC susceptibility in pipeline steels depends on various factors such as t[1]he microstructure of steel, distribution of inclusions and precipitates inside the steel, texture and micro-texture of steel, chemical composition of steel, pH of the oil and gas which is transported, the pH of soil and environment where the pipeline steel is buried, and many other factors. Importance of the SCC in pipeline failure motivated us to write this review paper. This paper concentrates on different factors affecting the SCC crack nucleation and propagation in pipeline steel and looks for new ways to increase the resistance of pipeline steels to the SCC.
2. Explanation of SCC and HIC
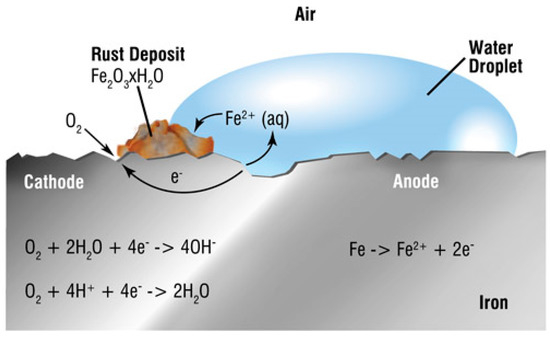
References
- Z.Y. Liu; X.G. Li; C.W. Du; L. Lu; Y.R. Zhang; Y.F. Cheng; Effect of inclusions on initiation of stress corrosion cracks in X70 pipeline steel in an acidic soil environment. Corrosion Science 2009, 51, 895-900, 10.1016/j.corsci.2009.01.007.
- Xian-Bo Shi; Wei Yan; Wei Wang; Lian-Yu Zhao; Yi-Yin Shan; Ke Yang; HIC and SSC Behavior of High-Strength Pipeline Steels. Acta Metallurgica Sinica (English Letters) 2015, 28, 799-808, 10.1007/s40195-015-0257-1.
- T. Hara; H. Asahi; H. Ogawa; Conditions of Hydrogen-Induced Corrosion Occurrence of X65 Grade Line Pipe Steels in Sour Environments. CORROSION 2004, 60, 1113-1121, 10.5006/1.3299225.
- Xian-Bo Shi; Wei Yan; Wei Wang; Lian-Yu Zhao; Yi-Yin Shan; Ke Yang; Effect of Microstructure on Hydrogen Induced Cracking Behavior of a High Deformability Pipeline Steel. Journal of Iron and Steel Research International 2015, 22, 937-942, 10.1016/s1006-706x(15)30093-5.
- Wan Keun Kim; Seong Ung Koh; Boo Young Yang; Kyoo Young Kim; Effect of environmental and metallurgical factors on hydrogen induced cracking of HSLA steels. Corrosion Science 2008, 50, 3336-3342, 10.1016/j.corsci.2008.09.030.
- Joseph Maciejewski; The Effects of Sulfide Inclusions on Mechanical Properties and Failures of Steel Components. Journal of Failure Analysis and Prevention 2015, 15, 169-178, 10.1007/s11668-015-9940-9.
- M.A. Mohtadi-Bonab; J.A. Szpunar; Seyed Salman Razavi-Tousi; A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Engineering Failure Analysis 2013, 33, 163-175, 10.1016/j.engfailanal.2013.04.028.
- M. A. Mohtadi-Bonab; M. Eskandari; R. Karimdadashi; J. A. Szpunar; Effect of different microstructural parameters on hydrogen induced cracking in an API X70 pipeline steel. Metals and Materials International 2017, 23, 726-735, 10.1007/s12540-017-6691-z.
- Zhou Fan; Xiaogang Hu; Jianyi Liu; Hongchuan Li; Jinwen Fu; Stress corrosion cracking of L360NS pipeline steel in sulfur environment. Petroleum 2017, 3, 377-383, 10.1016/j.petlm.2017.03.006.
- M. A. Mohtadi-Bonab; M. Eskandari; H. Ghaednia; S. Das; Effect of Microstructural Parameters on Fatigue Crack Propagation in an API X65 Pipeline Steel. Journal of Materials Engineering and Performance 2016, 25, 4933-4940, 10.1007/s11665-016-2335-6.
- M.A. Arafin; J.A. Szpunar; Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Materials Science and Engineering: A 2011, 528, 4927-4940, 10.1016/j.msea.2011.03.036.
- M.A. Arafin; J.A. Szpunar; A new understanding of intergranular stress corrosion cracking resistance of pipeline steel through grain boundary character and crystallographic texture studies. Corrosion Science 2009, 51, 119-128, 10.1016/j.corsci.2008.10.006.
- J. Wright; Inhibiting rust and corrosion to prevent machine failures. In Proceedings of the Machinary Lubrication Conference and Exhibition 2018, 1, 1-10, No DOI.
- National Energy Board.; Stress Corrosion Cracking on Canadian Oil and Gas Pipelines. National Energy Board. 1996, 1, 1-10, No DOI.
- O.F. Aly; M.M. Neto; Stress Corrosion Cracking, Developments in Corrosion Protection. IntechOpen Limited: London, UK 2014, 1, 1-10, No DOI.
- J. M. Sutcliffe; R. R. Fessler; W. K. Boyd; R. N. Parkins; Stress Corrosion Cracking of Carbon Steel in Carbonate Solutions. CORROSION 1972, 28, 313-320, 10.5006/0010-9312-28.8.313.
- John A. Beavers; Brent A. Harle; Mechanisms of High-pH and Near-Neutral-pH SCC of Underground Pipelines. Journal of Offshore Mechanics and Arctic Engineering 2001, 123, 147-151, 10.1115/1.1376716.
- E. A. Charles; R. N. Parkins; Generation of Stress Corrosion Cracking Environments at Pipeline Surfaces. CORROSION 1995, 51, 518-527, 10.5006/1.3294372.
- B. Y. Fang; Andrej Atrens; J. Q. Wang; E. H. Han; Z. Y. Zhu; W. Ke; Review of stress corrosion cracking of pipeline steels in “low” and “high” pH solutions. Journal of Materials Science 2003, 38, 127-132, 10.1023/a:1021126202539.
- M. A. Mohtadi-Bonab; H. Ghesmati-Kucheki; Important Factors on the Failure of Pipeline Steels with Focus on Hydrogen Induced Cracks and Improvement of Their Resistance: Review Paper. Metals and Materials International 2019, 1, 1-26, 10.1007/s12540-019-00266-7.
- M.G. Kadhim; M. Albdiry; A critical review on corrosion and its prevention in the oilfield equipment. Journal of Petroleum Research & Studies 2017, 14, 162-189, No DOI.
 Encyclopedia
Encyclopedia



