- Cyclodextins
- Flavors
- Food Additives
- Preservation
- Conservation
J.C. Mejuto[1], J. Simal-Gándara
Many of flavour responsible molecules used as food additives are volatile liquids subject to oxidation.[2][3] Cyclodextrins (CDs) are cyclic α-D-glucopyranose oligomers.[4] They arenon-toxic compounds, and, for this reason, they can be applied as technical additives for the protection of flavours, vitamins and colorants naturally present in food.[5][6] Their application in food engineering and food technology can be by different techniques[7][8] to avoid the formation of inclusion complexes between CDs and aromas for their use as flavour carrier.[9][10] These host-guest complex would reduce volatiziation losses or prevent oxidative decompositionp rocesses, improving organoleptic and nutritional properties.[11][12][13][14] Indeed, CDs can are empty capsules as we shown in Figure 1 where the volumes of every α, β and γ-CD is depict.
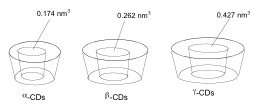
CDs and CDs derivatives with several inner cavity diameters are available and we can chose one of them as a function of guest molecules, molecular protection objective or technological use for the inclusion comples. Hence, α-CD normally form insoluble complexes with fat acids as guest molecule which is a clear advantage for clinical diagnose purposes.[15] Whereas, unsaturated fat acids form more stable host-guest complexes with β-CD that could interfere upon the brain fat acids equilibrium.[16] However, it is important to note that CDs supplied via oral or intravenous are unable to traverse bood-brain barrier.
The use of CD derivatives allows us to obtain highly soluble inclusion complexes. Replacing one or more hydroxyl groups does obtaining these derivatives from the CDs, which causes significant modifications in terms of the hydrophobicity of the cavity or its geometry. Those CDs of design together with the inclusion complexes formed with different molecules of interest would allow us to reach a higher level of stability for storage until its final decomposition in their biological medium.[17] Undoubtedly, one of the molecules that can be used for the formation of host-guest complexes for technological purposes are the aromas and fragrances for use in the food industry under conditions that allow greater stability by reducing the amount of food additive and increasing its efficiency over time.[18] In addition, they exhibit a high stability against heating in the different processes of transformation in industry and stay longer in the food when applied through traditional methods.[19][20]
In the literature it has been demonstrate the positive effect of host-guest complex formation. An exemplary case is that of Citral/Lactose and Cinnamaldehide/Lactose systems.[21][22] In this way, residual Citral/Lactose and Cinnamaldehide/Lactose systems flavour content of decreases 100% and ~80% respectively after 48 hours, while the residual flavour content of Citral/β-CD and Cinnamaldehide/β-CD systems decreases ~10% and ~30% respectively and, also, prevents the loss of flavour during the drying process. Moreover, the flavour/β-CD inclusion complex yields an effective protection of flavour even at 175-250 ºC and in isthoermic heat at 60ºC for 2-weeks threatments. It has also been shown that inclusion complexes formation protect the flavour effectively against UV radiation. In summary, the molecular encapsulation of these flavour concentrates resulted in remarkable improvement of stability during a long-term storage.[23] The remained flavour load of the β-CD complexes upon a long-term storage under normal conditions (14 years) are in the range of 40-100% for all flavours studied.
Finally, CDs act as secondary antioxidant in association with antioxidants (i.e. ascorbic acid). The system exhibits a synergic phenomena, which results in that system antioxidant capacity is preserved or, even improved.[24]
References
- Departamento de Química Física, Facultad de Ciencias, Universidad de Vigo, Campus de Ourense, 32004-Ourense, SPAIN
- José M. López-Nicolás; Estrella Núñez-Delicado; Francisco Garcia-Carmona; Álvaro Sánchez-Ferrer; Kinetic model of apple juice enzymatic browning in the presence of cyclodextrins: The use of maltosyl-β-cyclodextrin as secondary antioxidant. Food Chemistry 1970, 101, 1164-1171, 10.1016/j.foodchem.2006.03.018.
- José M. López-Nicolás; Antonio J. Pérez-López; Ángel A. Carbonell-Barrachina; Francisco Garcia-Carmona; Use of Natural and Modified Cyclodextrins as Inhibiting Agents of Peach Juice Enzymatic Browning. Journal of Agricultural and Food Chemistry 1970, 55, 5312-5319, 10.1021/jf070499h.
- Gonzalo Astray; Juan C. Mejuto; Jorge Morales; R. Rial-Otero; Jesus Simal-Gandara; Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Research International 1970, 43, 1212-1218, 10.1016/j.foodres.2010.02.017.
- Gonzalo Astray; C. Gonzalez-Barreiro; Juan C. Mejuto; R. Rial-Otero; Jesus Simal-Gandara; A review on the use of cyclodextrins in foods. Food Hydrocolloids 1970, 23, 1631-1640, 10.1016/j.foodhyd.2009.01.001.
- Gonzalo Astray; Antonio Cid-Samamed; L. García-Río; Carlos Lodeiro; Juan C. Mejuto; Óscar A. Moldes Figueiral; Jorge Morales; Cyclodextrin-Surfactant Mixed Systems as Reaction Media. Progress in Reaction Kinetics and Mechanism 1970, 35, 105-129, 10.3184/146867810x12686717520194.
- Jorge Morales; José Antonio Manso; Juan C. Mejuto; Basic hydrolysis of carbofuran in the presence of cyclodextrins. Supramolecular Chemistry 1970, 24, 399-405, 10.1080/10610278.2012.688121.
- R. K. Finn; Key note lecture: Food and biotechnology. Food Biotechnology 1970, 4, 1-13, 10.1080/08905439009549715.
- E.M.Martin Del Valle; Cyclodextrins and their uses: a review. Process Biochemistry 1970, 39, 1033-1046, 10.1016/s0032-9592(03)00258-9.
- Lajos Szente; Jozsef Szejtli; Cyclodextrins as food ingredients. Trends in Food Science & Technology 1970, 15, 137-142, 10.1016/j.tifs.2003.09.019.
- É. Fenyvesi; M. A. Vikmon; L Szente; Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations in 2012. Critical Reviews in Food Science and Nutrition 1970, 56, 1981-2004, 10.1080/10408398.2013.809513.
- Eva Fenyvesi; Lajos Szente; Nanoencapsulation of flavors and aromas by cyclodextrins. Encapsulations 1970, 2, 769-792, 10.1016/b978-0-12-804307-3.00018-1.
- Paweł K. Zarzycki; Boz˙ Ena Fenert; Bronisław K. Głód; Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. Encapsulations 1970, 2, 717-767, 10.1016/b978-0-12-804307-3.00017-x.
- Joana F. Fangueiro; Eliana B. Souto; Amélia M. Silva; Encapsulation of nutraceuticals in novel delivery systems. Nutraceuticals 1970, 4, 305-342, 10.1016/b978-0-12-804305-9.00009-9.
- Jozsef Szejtli; Utilization of cyclodextrins in industrial products and processes. Journal of Materials Chemistry 1970, 7, 575-587, 10.1039/a605235e.
- D Sun; D D Gilboe; Effect of the platelet-activating factor antagonist BN 50739 and its diluents on mitochondrial respiration and membrane lipids during and following cerebral ischemia.. Journal of Neurochemistry 1970, 62, 1929-1938, 10.1046/j.1471-4159.1994.62051929.x .
- J. Szejtli; Selectivity/structure correlation in cyclodextrin chemistry. Supramolecular Chemistry 1970, 6, 217-223, 10.1080/10610279508032537.
- T.A. Reineccius; G.A. Reineccius; T.L. Peppard; Flavor Release from Cyclodextrin Complexes: Comparison of Alpha, Beta, and Gamma Types. Journal of Food Science 1970, 68, 1234-1239, 10.1111/j.1365-2621.2003.tb09631.x.
- L. Szente; J. Szejtli; Stabilization of Flavors by Cyclodextrins. ACS Symposium Series 1970, 370, 148-157, 10.1021/bk-1988-0370.ch016.
- Szejtli, J.. Cyclodextrin Technology; Springer Science & Business Media: Berlin, 2013; pp. 1-450.
- Gerhard Wenz; Cyclodextrins as Building Blocks for Supramolecular Structures and Functional Units. Angewandte Chemie International Edition in English 1970, 33, 803-822, 10.1002/anie.199408031.
- Xiang-Dong Liu; Takeshi Furuta; Hidefumi Yoshii; Pekka Linko; W. Jan Coumans; Cyclodextrin Encapsulation to Prevent the Loss of l -Menthol and its Retention during Drying. Bioscience, Biotechnology, and Biochemistry 1970, 64, 1608-1613, 10.1271/bbb.64.1608.
- T.A. Reineccius; G.A. Reineccius; T.L. Peppard; Encapsulation of Flavors using Cyclodextrins: Comparison of Flavor Retention in Alpha, Beta, and Gamma Types. Journal of Food Science 1970, 67, 3271-3279, 10.1111/j.1365-2621.2002.tb09577.x.
- N. Mantilla; M.E. Castell-Perez; C. Gomes; Rosana G. Moreira; Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus). LWT - Food Science and Technology 1970, 51, 37-43, 10.1016/j.lwt.2012.10.010.
 Encyclopedia
Encyclopedia



