Subjects:
Physics, Applied
1. Introduction
The demand for fossil fuels is anticipated to grow 40% from 2010 to 2040 [1]. Therefore, to satisfy our energy needs, alternative energy sources have been, and are being, explored. Solar, wind and biomass are the major renewable energy sources. Biomass, derived from a biological precursor, has been used to produce biofuels and bioproducts over the last few decades [2]. Depending on type of biomass, there are first to fourth biofuel generations. Biofuels include biodiesel, bioethanol, biomethanol, biohydrogen, and bioethers (biodimethyl ether, biomethyltetrabutyl ether (bio-MTBE)) and bioethyltetrabutyl ether (bio-ETBE) [3]. According to the U.S. Department of Energy, the major biofuels are bioethanol and biodiesel, both of which represent the first generation of biofuel technology [4]. Several biofuel projects have been funded by U.S.A., Australia and European Union. The United States funded projects in Arizona (2008), New Mexico (2009), Massachusetts (2011) and Florida (2013) while the European Union funded four pilot projects; three of which were from 2011 to 2015/16 and the fourth was from 2012 till 2017 [5].
Agrofuel is the first biofuel generation that used specific cultivated plants including sugarbeet, sugarcane, maize, palm, soybean, and sweet sorghum as feedstocks for production. Agrofuel is produced through yeast fermentation of plant sugars or starch to give bio-ethanol and the extracted plant oils to produce biodiesel [6]. These processes greatly negatively impact both the food and water sectors [7]. Second generation biofuels depended on non-food plants like Jatropha, grass, switchgrass, silver grass and non-edible parts of current crops [8]. In order to reduce land and water utilization and excessive use of harmful pesticides [2], algal biofuel emerged as the third generation of biofuels with no competition [9]. The different types of algal biofuels are summarized in Figure 1. The fourth biofuel generation is focused on metabolic engineering of the microalgal genome to maximize the biofuel yields or minimize the cost [6,10]. The leaders in biofuel development and consumption are Brazil, United States, France, Sweden and Germany [11]. Recently, several studies have shown promising results in increasing carbon capture capacity, biomass production, and lipid enhancement in genetically modified microalgae [10,12,13,14].
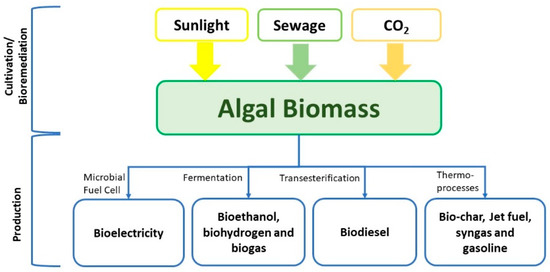
Figure 1. Various scenarios and conversion processes of algae for biofuel production.
2. Algae
Algae are aquatic organisms with different species that range in size from microalgae to macroalgae [15] (Figure 2).
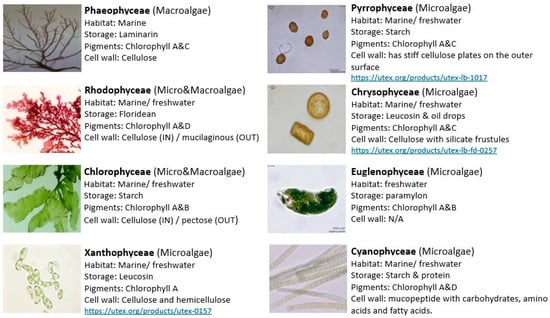
Figure 2. Different groups of algae. Various options are available for algae type and strain choice.
They differ in shapes from a single cell to multicellular structures like filaments and colonies. Most algae can habit any imaginable environment and can withstand extreme conditions [16]. Algae are classified by their development to cyanoprokaryotes and eukaryotes. Cyanoprokaryotes are characterized by the lack of a well-defined nucleus and chloroplasts. Eukaryotic algae are further divided according to cell wall structure, pigments, and storage products into Chlorophyceae, Phaeophyceae, Rhodophyceae, Xanthophyceae, Pyrrophyceae, Euglenophyceae and Chrysophyceae. Unlike higher plants, algae have no embryo, vascular tissues or surrounding layer around sex organs. The economic importance of algae is evident in wastewater treatment, production of energy cogeneration, bioremediation, natural fertilizer, animal fodders, medicinal compounds and nutraceuticals. In addition, numerous products can be obtained from algae including proteins, vitamins, pigments, nutraceuticals and special oils (omega-3) [5]. Algae can use light more efficiently, grow faster and can produce 2–15 fold higher lipids than higher plants like Jatropha, rapeseed and soybean [17,18,19,20].
2.1. Microalgal Cultivation
Algae possess high carbon dioxide sequestering efficacy, high photosynthetic levels and high growth rates. They also use nitrogen and phosphorous from municipal, agricultural, and industrial wastewater which reduces the nutrient load in wastewater. They contain substantial amounts of lipids that can produce nontoxic and highly biodegradable biofuels. Algal cultivation for biofuel production sounds quite simple since algae have relatively simple requirements to grow. However, several factors affect the optimum algal growth and lipid accumulation including micro- and macronutrients (availability and concentration), CO2, temperature, pH, and light (intensity and photoperiod). Algae differ in their reaction to these conditions especially to temperature and light. A temperature between 20–30 °C is considered suitable for most algal species. The quantity of unsaturated fatty acids decreases with increasing the temperature which is a physiological adaptation [21]. In addition, the type of precursor fatty acids influences several biodiesel properties especially oxidative stability, melting point, heating point, iodine and cetane numbers, and lubricity. The usual targets for qualified biodiesel production are palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) [22]. Under limiting conditions, some microalgae alter their lipid biosynthesis pathway to make large quantities of neutral lipids (20–50% of dry weight) typically as triacylglycerol (TAG) and primarily stored in cytosolic lipid bodies [23].
For biofuel production, it is better to survey a large number of algal species first then select the species with higher productivities and optimize all conditions to reach maximum production for these species. Hundreds of experiments can be performed for one species to maximize both its biomass and biofuel production. The selection and optimization processes are usually carried out in a bulk-culture in vitro. Because the conventional lab techniques (Figure 3A) are labor-intensive and time consuming, microfluidics or on-chip technology found its importance. Algae-on-chip relies on estimating a droplet as a vessel of culturing starting with a single cell per droplet (Figure 3B). One of the remarkable advantages of this technique is that the microfluidic device captures more than 100 droplets at once (this number can be increased according to the design). These hundred droplets captured in the same device represent 100 replicates in a space less than 4 × 4 cm which is impossible to accomplish by the traditional methods. Different designs can be fabricated to test different culture conditions, and even to extract oil, and DNA. Microfluidics are time and labor saving with high through-put single cell analysis. This technique also allows to test more than one factor rapidly. Despite having some defects, this technique was able to overcome all the disadvantages of the previous techniques [24]. Microfluidic chips have been used for several applications including ecotoxicology screening [25], cell identification [26], cultivation under multiple conditions, lipid analysis [27], sorting [28], trapping, cell viability [29], and quantify self-secreted macromolecules e.g., ethanol [30] and lactate [31].
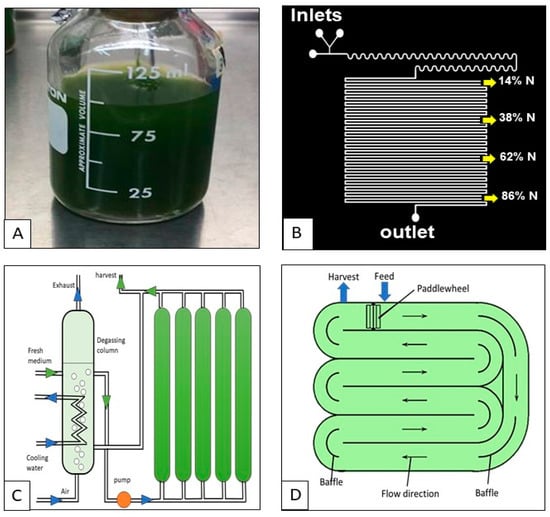
Figure 3. Algal cultivation techniques. (A) Conventional method (in vitro), (B) Lab on-a-chip method (in vitro), (C) Photobioreactor (in vivo), and (D) Open pond (in vivo).
Microalgae cultivation is a significant factor in biofuel production. Cultivation system selection is particularly important since it affects the phytoremediation efficiency and biofuel yield. Generally speaking, photoautotrophic systems can be closed and open. Closed systems (photobioreactors (PBRs)) are highly-controlled, high-yield systems intended to allow high light accessibility and perfect stirring [32,33] (Figure 3C). Photobioreactors come in different designs including the flat plate, tubular, or columns, with tubular being the most common [34]. They can be constructed as plastic or glass bags, tanks, or towers. Bubble columns and airlift photobioreactors were reported to give a comparatively higher microalgal biomass. Since excessive oxygen can negatively affect the algal growth, an auxiliary tank is usually added to separate it [19]. While contamination is eliminated [35], the major drawback of these controlled facilities is their cost [34]. Other challenges include overheating, oxygen buildup, difficulty of scaling-up, bio-fouling, and overtime cell damage [16].
On the other hand, open systems (also called open ponds) are less expensive, but less controlled, than the closed systems (Figure 3D). The most commonly used forms are the raceway pond, the circular pond tank, the closed pond, and the shallow big pond. One of the main features of open systems is their ability to utilize the atmospheric CO2. Location of the open system is a very important criterion since it affects sunlight availability. Also, they are usually equipped with a rotating arm to guarantee continuous stirring of the culture [34]. Contamination by bacteria or even other microalgae is the major challenge of these systems [36]. Other drawbacks include poor light penetration, unregulated temperature, evaporation loss, and CO2 diffusion into the atmosphere [16]. However, this system is more affected by variability in light, water temperature, and evaporation. When deciding between these two systems, the comparison is mainly based on the productivity per unit area and the cost. Photobioreactors have higher productivity rates than open ponds due to the controlled growth conditions which ensures good light penetration. Nevertheless, open ponds are cheaper than the closed systems that require infrastructure, operation, and maintenance costs [37]. One of the main advantages of using photoautotrophic systems is the consumption of carbon dioxide as the carbon source. However, if CO2 is used as the only carbon source, it is desirable to have the cultivation as close as possible to facilities that can provide adequate amounts of CO2. Moreover, compared to other cultivation methods, contamination is less severe when using photoautotrophic systems [38].
A setup combining an open system and a closed system is known as a hybrid system. Hybrid systems accomplish excellent biomass productivity along with high nutrient removal [39]. They are designed to overcome the huge initial and operating costs of closed systems and the limitations of open systems. In hybrid systems, microalgae are cultured in a closed photobioreactor first then transferred to an open system to enhance the yield [40]. Hybrid systems are appropriate for large algal cultivation [19].
According to their metabolic pathways, algae can be autotrophic, heterotrophic, mixotrophic, and photoheterotrophic [41]. Autotrophic pathway or photosynthesis involves the conversion of inorganic carbon into organic energy in the presence of light [42]. Heterotrophic pathway needs organic carbon to feed in the dark [43] whereas, in mixotrophic pathway, cells can grow autotrophic or heterotrophic based on the available food sources [44]. Photoheterotrophic pathway takes place in the presence of light and organic carbon [42]. Heterotrophic metabolism results in a higher growth rate compared to autotrophic metabolism [45]. It has been reported that mixotrophic metabolism is the best way to get maximum biomass and lipid productivities [46].
Algal Fuels
As the third generation feedstock, microalgae have a huge potential for biofuel production due to their quick growth, great biomass yield, and high lipid and carbohydrate contents. Biodiesel, biogas, bioethanol, and biomethane are among the valuable biofuels produced by algae. Algal carbohydrates are used for producing bioethanol, while algal oils are used for biodiesel production. The remaining biomass is used for methane or fuel oil production. After biofuel production, the residual biomass can be used to produce nutraceuticals, protein supplements, therapeutics, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), biocontrol agents, fertilizers, and animal feed. Biodiesel is a biodegradable fuel that reduces sulfur and particulate matter emissions while having engine performance similar to petroleum [56]. Biogas or biomethane is produced through the anaerobic digestion of organic matter. Biogas is chiefly made of methane (65–75%) and carbon dioxide (25–35%) [57]. The anaerobic digestion process involves (1) biopolymer hydrolysis by hydrolytic bacteria to monosaccharaides, (2) conversion of the monosaccharaides into acids through fermentation, (3) formation of acetate by action of acetogenic bacteria, and (4) formation of methane and carbon dioxide by methanogenic bacteria [58]. Microalgal hydrocarbons can be converted to kerosene, diesel, and gasoline. For example, Botryococcus braunii produces hydrocarbons with excellent oil yield outside the cell which is more convenient for extraction [59]. In the presence of air, oxygen, or water vapor, bio-syngas is produced by the biomass gasification to give methane, hydrogen, carbon monoxide, water and ashes [60]. The gasification process requires a high temperature of 800–1200 °C. It is desirable that the biomass water content is below 20% [61]. In absence of oxygen, microalgae can directly produce hydrogen, as a promising source of clean energy that does not emit greenhouse gasses, from sunlight and water [62]. Bioethanol is obtained from fermenting sugars by yeast. Some microalgae were reported to have starch content above 50% [63,64]. The yield of polysaccharides in seaweeds was 3.6–11.7 g/L and 179–260 mg/g [65]. Microalgal cellulose and hemicellulose can be converted to sugars and then ethanol [66].
2.4. Conversion Techniques
Algae are significant candidates for biofuel production [23,103,104]. Different products can be obtained from algae according to species type, cultivation system and processing of biomass. Energy products from algae include biodiesel, biokerosene (jet fuel), gasoline, ethanol, methanol, hydrogen and syngas [5]. Algae can be processed in different ways to create an array of end-use energy products. After harvesting, algal biomass can be processed through thermochemical [105], biochemical, transesterification, and photosynthetic microbial fuel cell conversion processes [106].
2.4.1. Thermochemical Conversion
Thermochemical conversion processing involves the thermal breakdown of biomass then organic chemical reformation into biofuels through pyrolysis, gasification, combustion or hydrothermal liquefaction [2,106]. Pyrolysis is a thermal decomposition of biomass to produce solid fuel (biochar), liquid fuel, and gaseous fuel products in the absence of oxygen [16]. Pyrolysis could be slow (a slow heating rate of 0.1–1 °C/s for long duration), fast (a fast heating rate of 10–200 °C/s in a short duration), or flash (a very fast heating rate of >1000 °C/s for a very short duration). Typically, it happens at a rate of 300–700 °C/s [29,107,108]. Taking into consideration the high ash content of algae, pyrolysis is the most preferred conversion process but the produced oil still has some issues with its acidity, viscosity, and stability [16]. The microwave enhanced pyrolysis (MEP) has been recommended as a quick, efficient method for bio-oil production [109]. Gasification is the partial oxidation of algal biomass with a controlled quantity of oxygen, steam or air at 700–1000 °C [106] that results in a syngas (a mixture of different gases mainly H2, CO, CO2, and CH4) [2]. Direct combustion involves oxygenation of biomass in a boiler, furnace, or steam turbine at a temperature around 1000 °C to produce hot gases. Prior to the combustion stage, pre-treatments like drying and grinding into smaller particles are required [16]. In the hydrothermal liquefaction, algal slurries are exposed to 300–400 °C and 40–200 bar to produce biocrude, gas, and char (10–73, 8–20, and 0.2–0.5%, respectively) [110,111,112,113,114]. Several compounds can be extracted or depolymerized from the algal biomass via liquefaction [115]. Oil yield from hydrothermal liquefaction is in the range of 9-97% [116,117] which is higher than the pyrolysis bio-oil [118].
2.4.2. Biochemical Pathways
The biochemical pathway of conversion involves hydrolysis of cell walls by bacteria into fermentable sugars [2]. Fermentation refers to the anaerobic digestion of sugars into biogas, bioethanol, or biohydrogen. Biogas is produced through acetogenesis in which all the fermentable products are oxidized into acetate which is converted during methanogenesis into methane and CO2 [16]. Biogas production is influenced by carbon nitrogen (C:N) ratio in the feedstock, duration, temperature, pH, solids, and feeding rates [2]. The biogas yield is rather small due to the algal sensitivity to degradation by bacteria and low C:N ratio, which results in the production of ammonia (inhibitor). Interestingly, the lipid-free and amino acid-free residual biomass of Scenedesmus spp. gave better biogas yield compared to the raw one [119]. To overcome the lower C:N ratio, the biomass is usually co-digested with waste papers and sewage sludge [36,120]. This co-digestion was reported to increase the CH4 production by 26% [121]. The use of salt-adapted micro-organism was reported to attenuate the effect of high protein content on anaerobic digestion [34]. Microwave pre-treatment of biomass can increase biogas yield by 56% via altering the cell wall structure [122]. Methane production can be enhanced by enzymatic, mechanical and thermal pretreatments [67,123].
Bioethanol is obtained from yeast fermentation of hydrolyzed carbohydrates [124]. Glycogen-containing cyanobacteria were examined for bioethanol production and resulted in 6.5 g/L and 350 mg/g [125]. Phaeophyceae is considered the most appropriate feedstock for bioethanol production owing to its high sugar content [33,126]. Pre-treatments like milling, hot water wash, liquefaction, enzymatic hydrolysis, and saccharification or alginate extraction are essential for efficient bioethanol production [33,127,128]. Oxygen, as a photosynthesis byproduct, can suppress the hydrogenase pathway [129], nevertheless, anaerobic digestion can conquer this problem [130]. Interestingly, a significant increase in hydrogen production (20-fold) was reported in continuous flow regime compared to batch production [131].
2.4.3. Transesterification
Transesterification is the process wherein triglycerides react with an alcohol (commonly methanol or ethanol) in the presence of an acidic or a basic catalyst to give biodiesel and glycerol [132] (Scheme 1). The reaction highly depends on alcohol type, catalysts type, and molar ratio. This process is important to reduce algal oil viscosity and increase its fluidity in order to be mixed with petroleum diesel and applied directly to engines [16]. Conventional transesterification involves extraction of algal lipids then esterification whereas direct transesterification is a single-step method where the wet biomass is treated directly without extraction. Biodiesel yield from direct transesterification is usually much lower than the conventional methods although it saves reagents, energy, and time. To save energy, it is best to combine microwave and ultrasound irradiation techniques which will increase the yield to 90%. The use of in situ supercritical methanol transesterification method has been reported as another way to reduce the cost [133,134]. The main fatty acids used in producing qualified biodiesel are palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) [135]. Biodiesel properties are strongly influenced by its fatty acids content. Polyunsaturated fatty acids (PUFAs) are not favorable because of their susceptibility to oxidation, and saturated lipids tend to elevate the cloud point and viscosity of biodiesel [136], therefore, monounsaturated fatty acids (MUFAs) are the most desired lipids [137]. Heat of combustion, melting point, and viscosity of biodiesel increase with the length of the fatty acid chain and inversely correlated to unsaturation [136]. Autoxidation and lubricity increase with increasing unsaturation [138]. Lipase, as a catalyst in transesterification, catalyzes both esterification and transesterifications simultaneously and helps excluding byproduct recovery [139,140]. Chlorophytes, diatoms, and cyanobacteria with high lipid content have great potential in biodiesel production [141]. Oleaginous strains (at least 20% lipid content on dry weight basis) can overproduce lipids (up to 70% lipids on dry weight basis) under specific severe stress conditions such as nitrogen and/or silicon (Si) deprivation.
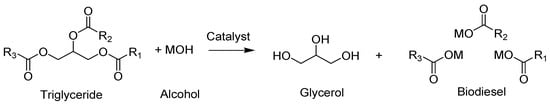
Scheme 1. A typical transesterification reaction.
2.4.4. Photosynthetic Microbial Fuel Cell
In recent years, microbial fuel cells (MFCs) were developed a result of the imminent energy crisis [142,143]. MFC activity depends on photosynthetic oxygen generated at cathode to cause increment in the electron transfer from the anode [144] (Figure 4). The limiting factor in traditional MFC is the mechanical aeration at the cathode, employed as a terminal electron acceptor (TEA). The application of algal photosynthesis at the cathode to substitute for the energy-intensive mechanical aeration was reported [145,146,147,148,149,150]. The synergy between bacterial fermentation at the anode and the oxygenic photosynthesis of microalgae at the cathode supports decent power output [142]. During the day, the algal photosynthetic activity also results in elevating dissolved oxygen (DO) concentration. DO contributes to enhancing the reduction reaction rates at the cathode, which in turn improves bio-electrogenic activity. During night-time, the small DO levels lead to a drop in power output [142]。
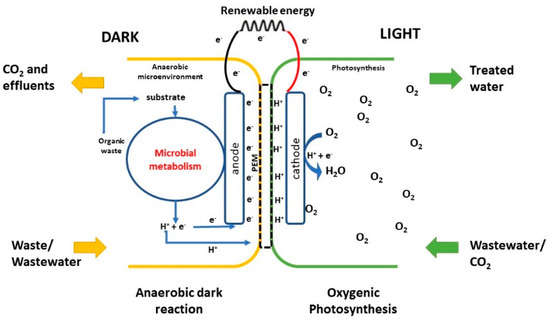
Figure 4. Diagram of a microbial fuel cell (MFC).
3. Genetic Engineering Toward Biofuels
Genetic engineering techniques, especially stable heterologous gene expression and genetic transformation, are required to advance algal biofuel production. Acetyl-CoA carboxylase (ACCase) catalyzes the first step in fatty acid synthesis, however, overexpression of this gene alone is not enough to enhance oil production in algae [151,152]. ACCase was overexpressed in Cyclotella cryptica which led to a slight increase in lipid content [151]. Interestingly, a significant increase in algal lipids was reported with the overexpression of diacylglycerol acyltransferase (DAGAT) [153]. In addition, C. reinhardtii was reported to accumulate a high lipid content if the metabolic pathways of starch had been blocked [154,155]. A similar result was observed for Chlorella pyrenoidosa[156]. It would be interesting to see how the lipid content would be affected by activating ACCase, activating DAGAT, blocking starch pathway, and increasing lipid synthesis.
Enhancement of hydrogen production via genetic engineering has been reported by decreasing the light antenna harvesting size, and inhibiting hydrogenase [157,158]. A mutant strain of Chlamydomonas reinhardtii(Stm6) with a blocked cyclic electron flow through Photosystem I (less competition for electrons; anaerobiosis), showed increased accumulation of starch and decrease intracellular concentrations of oxygen (hydrogenase inhibitor) [159]. Anaerobiosis can be achieved by a copper responsive nuclear transgene [160]. To overcome the light penetration limitation, a single RNAi construct was able to effectively silence all twenty light-harvesting complex (LHC) protein isoforms of C. reinhardtii increased light transmittance in the culture by 290% [161]. However, the modified cell density did not increase [162]. Growing algae under heterotrophic or mixotrophic settings results in greater cell densities, thus decreased harvesting cost [163]. While most algae are strict autotrophs [162], Volvox carteri, Phaeodactylum tricornutum, Cylindrotheca fusiformis and C. reinhardtii were effectively transformed with a hexose transporter (HUP1) causing glucose transport into the cells [164,165,166,167]. Complete and on-going projects for the whole genome identification of different algal species as C.reinhardtii, Thalassiosira pseudonana, and Micromonas pusilla provide information to understand algal behavior and structure [168]. Technological interventions of genetic and metabolic engineering, and synthetic biology have the potential to generate renewable fuel sources that do not compete with food industry or involve fresh water or agricultural land [169].
 Encyclopedia
Encyclopedia

