Recently, dental pulp stem cells (DPSCs) and stem cells from human exfoliated deciduous teeth (SHEDs) have attracted substantial attention as promising cell sources for tissue regeneration. Here, we summarized the features of DPSCs and SHEDs such as high growth capacity, multipotency, expression of cell markers, immunomodulatory effects, and their potential to regenerate various somatic tissues.
Mesenchymal stem cells (MSCs) have the capacity for self-renewal and multilineage differentiation potential, and are considered a promising cell population for cell-based therapy and tissue regeneration. MSCs are isolated from various organs including dental pulp, which originates from cranial neural crest-derived ectomesenchyme. Recently, dental pulp stem cells (DPSCs) and stem cells from human exfoliated deciduous teeth (SHEDs) have been isolated from dental pulp tissue of adult permanent teeth and deciduous teeth, respectively. Because of their MSC-like characteristics such as high growth capacity, multipotency, expression of MSC-related markers, and immunomodulatory effects, they are suggested to be an important cell source for tissue regeneration.
- dental pulp stem cells
- stem cells from human exfoliated deciduous teeth
- regenerative therapy
- regenerative medicine
1. Introduction
1
Mesenchymal stem cells (MSCs) have been reported to be isolated from various somatic tissues such as bone marrow [1], adipose tissue stromal vascular fraction[2], umbilical cord [3], and placenta [4]. They are noteworthy for their robust self-renewal capacity and multilineage differentiation potential including osteogenic, adipogenic, chondrogenic, myogenic, neurogenic, and tenogenic[5][6][7][8]. Thus, cell-based medicine using MSCs is expected to facilitate tissue regeneration in various somatic disorders. MSC-like populations have also been identified in dental tissues. Gronthos et al. initially identified dental stem cells from the human pulp tissue and termed dental pulp stem cells (DPSCs)[9]. Subsequently, dental MSC-like populations from other dental tissues were identified and characterized: Stem cells from human exfoliated deciduous teeth (SHEDs)[10], periodontal ligament stem cells (PDLSCs)[11], dental follicle precursor cells (DFPCs)[12], and stem cells from apical papilla (SCAPs)[13]. Among these stem cells, DPSCs and SHEDs are more attractive as cell sources for regenerative medicine than other MSC-like populations because of their easy accessibility.
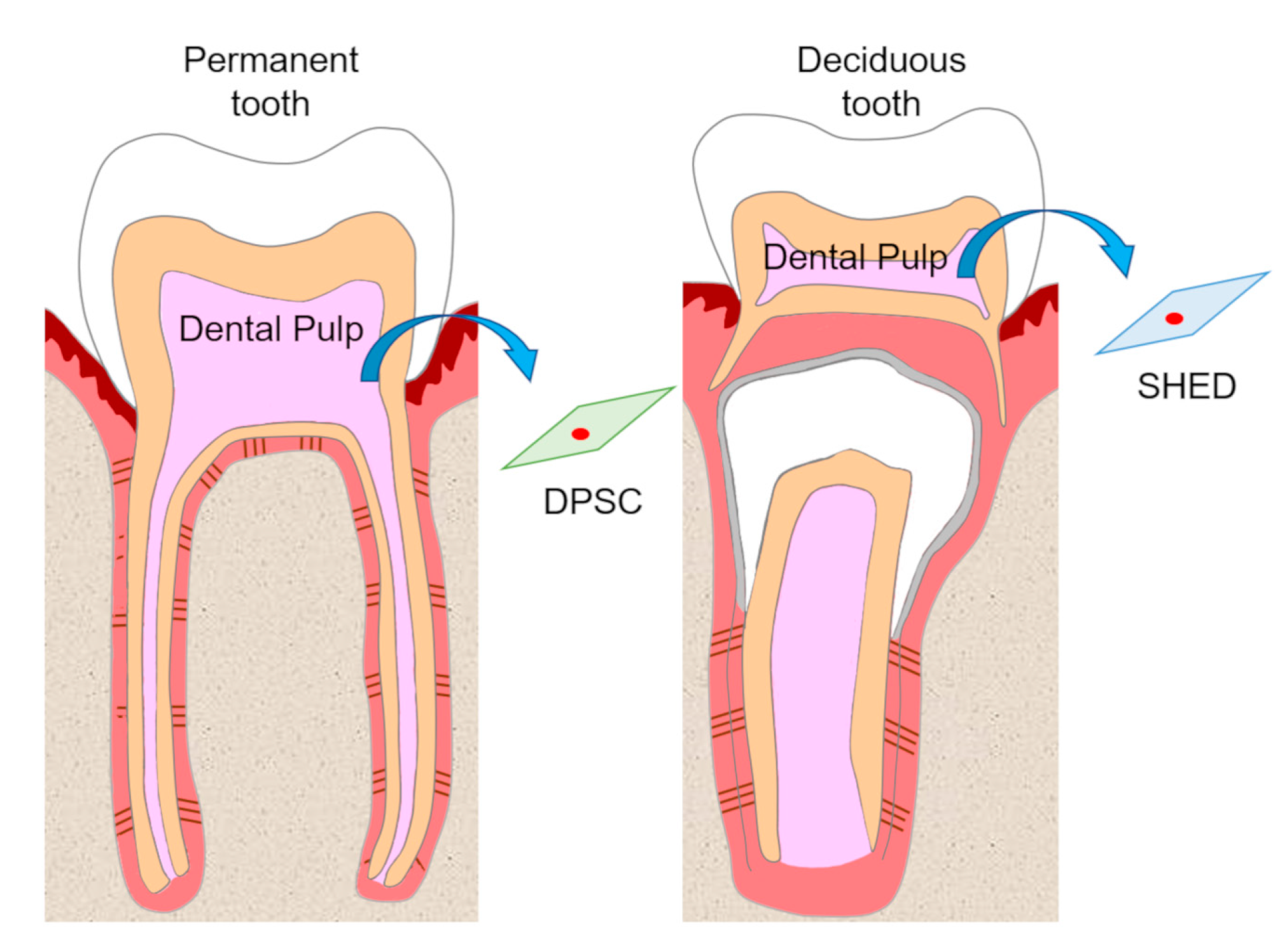
Dental pulp is an unmineralized connective tissue that is composed of stem cells as well as fibroblastic cells, capillary blood vessels, peripheral nerves, lymphatic elements, and extracellular matrices and odontoblasts on the periphery of pulp tissue. DPSCs are isolated from adult human pulp tissue of impacted third molars, orthodontic teeth, and supernumerary teeth, and have been characterized as cells with intense clonogenicity, proliferative activity, and the ability to form mineralized nodules[9] (Figure 1).
Figure 1. Isolation of dental pulp stem cells (DPSCs) and stem cells from human exfoliated deciduous teeth (SHEDs). DPSCs and SHEDs isolated from pulp tissue of permanent teeth and deciduous teeth, respectively, have the potential to be cell sources in regenerative medicine.
2. Self-Renewal and High Growth Capacity of DPSCs and SHEDs
The ability to self-renew is one of the essential defining features of stem cells. This ability is divided into two types according to how they self-renew. One involves generating daughter cells with developmental potential similar to that of the original stem cells (symmetric division), while the other involves generating both undifferentiated cells and cells fated to differentiate (asymmetric division)[14] (Figure 2). Consequently, stem cells can expand during development, be maintained in somatic tissues, and participate in tissue regeneration.
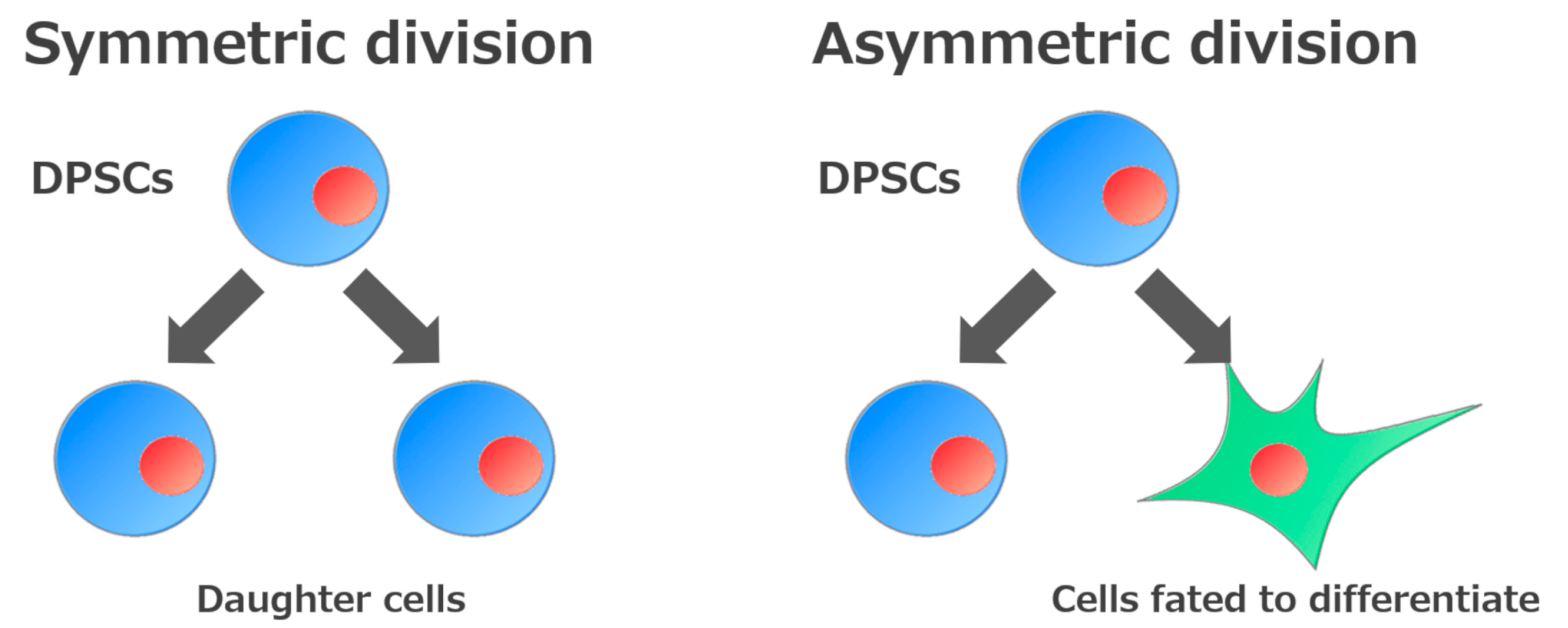 Figure 2. Self-renewal mechanisms of stem cells. Stem cells are defined by their ability to make new stem cells (self-renewal). Self-renewal of stem cells is divided into two processes: Generation of daughter cells or of cells fated to differentiate.
Figure 2. Self-renewal mechanisms of stem cells. Stem cells are defined by their ability to make new stem cells (self-renewal). Self-renewal of stem cells is divided into two processes: Generation of daughter cells or of cells fated to differentiate.
3. Multipotency of DPSCs and SHEDs
DPSCs and SHEDs have the ability to differentiate into various cell types under appropriate culture conditions (Figure 3).
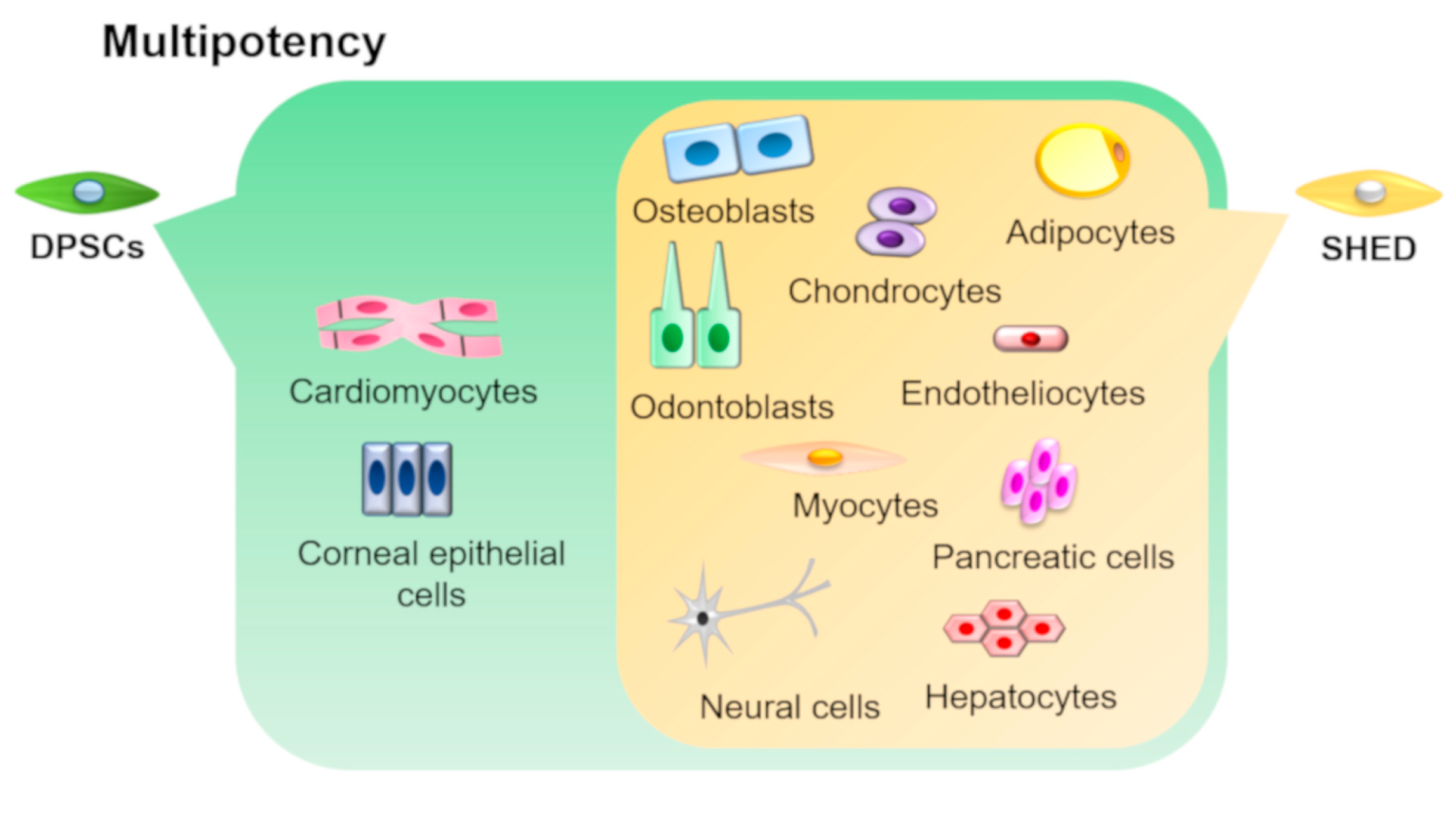 Figure 3. Multipotency of DPSCs and SHEDs. DPSCs and SHEDs can differentiate into multiple lineages such as osteoblasts, odontoblasts, adipocytes, chondrocytes, neural cells, endotheliocytes, myocytes, hepatocytes, and pancreatic cells under appropriate culture conditions. In addition, DPSCs can also differentiate into corneal epithelial cells and cardiomyocytes.
Figure 3. Multipotency of DPSCs and SHEDs. DPSCs and SHEDs can differentiate into multiple lineages such as osteoblasts, odontoblasts, adipocytes, chondrocytes, neural cells, endotheliocytes, myocytes, hepatocytes, and pancreatic cells under appropriate culture conditions. In addition, DPSCs can also differentiate into corneal epithelial cells and cardiomyocytes.
4. Semaphorin 3A
Semaphorin 3A (Sema3A), which is a member of the semaphorin family, was identified as an axonal guidance factor regulating nervous system development[19], and has been reported to play important roles in the development of various tissues such as blood vessels, peripheral nerves, and skeletal tissues[20][21][22]. In addition, Sema3A acts as an osteoprotective factor by inhibiting osteoclastic bone resorption and promoting osteoblastic bone formation[24]. Our previous study demonstrated that a multipotent human PDL cell line[23] highly expressed Sema3A compared with a PDL cell line with low differentiation potential (low-potent)[25]. In addition, we generated a Sema3A-overexpressing low-potent PDL cell line and clarified that these cells exhibited enhanced capacity to differentiate into osteoblasts and adipocytes. Moreover, Sema3A-overexpression enhanced self-renewal ability and increased the expression levels of markers of pluripotency, such as Oct-4, Nanog, and E-cadherin, and; MSC-related markers, such as CD73, CD90, CD105, CD146, and CD166[26]. Our results indicate that Sema3A regulates the stem cell features of PDLSCs and induction of this gene provide multipotency for a low-potent PDL cell line. Yamada et al. reported that Sema3A knockdown decreased self-renewal ability and the expression of Sox2, Oct-4, and CD44 in lung cancer stem cells. Their results support our hypothesis that Sema3A would play an important role in acquisition and maintaining of stemness.
Recently, we demonstrated that Sema3A increased migration, proliferation, and odontoblastic differentiation of DPSCs (Figure 4). Moreover, Sema3A facilitated reparative dentin formation after direct pulp capping in a rat pulp exposure model. These results suggested that Sema3A can recruit latent DPSCs to the wounded site and increase its biological activities to regenerate dentin/pulp complex. At the present time, we are planning to clarify the precise mechanisms of epigenetic regulation by Sema3A and to confirm the possibility for its use as a therapeutic agent that could increase the activity of hosts’ latent progenitor cells, especially DPSCs.
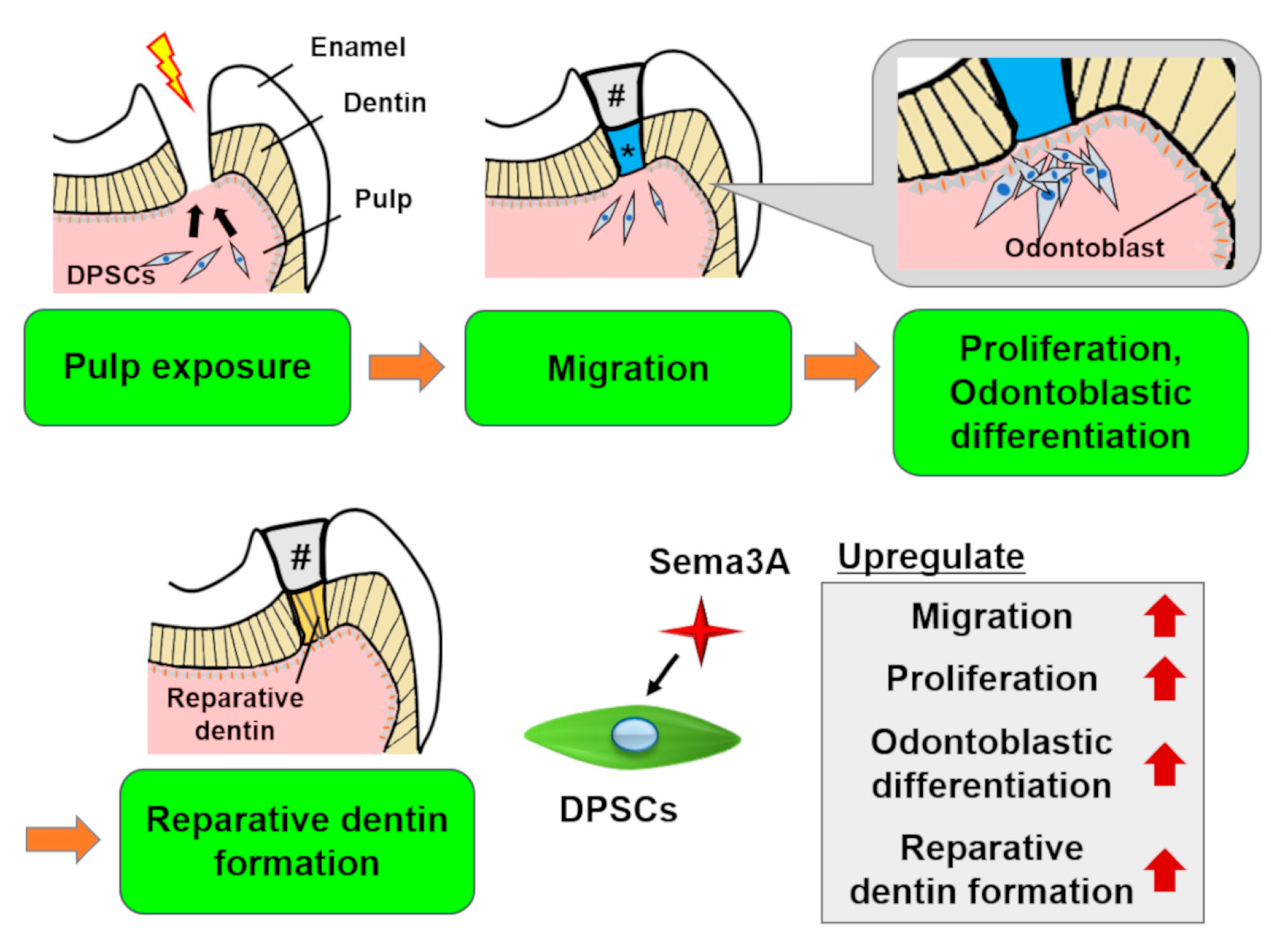 Figure 4. Schema of the effects of Semaphorin 3A (Sema3A) on DPSCs. In case of pulp exposure, DPSCs migrate toward the exposure site, then they proliferate and differentiate into odontoblasts to form reparative dentin. Sema3A can act as a bioactive agent to facilitate these regenerative processes. #, sealing material; *, Sema3A.
Figure 4. Schema of the effects of Semaphorin 3A (Sema3A) on DPSCs. In case of pulp exposure, DPSCs migrate toward the exposure site, then they proliferate and differentiate into odontoblasts to form reparative dentin. Sema3A can act as a bioactive agent to facilitate these regenerative processes. #, sealing material; *, Sema3A.
 Encyclopedia
Encyclopedia