1000/1000
Hot
Most Recent

Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the cause of the current Coronavirus Disease 2019 (COVID-19) pandemic, whose first case was reported in December 2019 in Wuhan, Hubei province, China. In January 2021, the pandemic is still ongoing and is getting worse [1]. Dental surgery is considered to be a profession at high risk for being infected, and therefore transmitting SARS-CoV-2. Our professional practice was disrupted by lockdowns, resulting in reduced activity, new dental protocols and additional costs for staff protective equipment. This has caused unexpected financial difficulties for many dental practitioners. Even with treatments or vaccines, our professional practice will probably never revert back to the previous situation, as the new constraints may become permanent.
The oral cavity can be a significant reservoir for respiratory pathogens such as Mycobacterium tuberculosis, Influenza virus, SARS-CoV, MERS-CoV, but also SARS-CoV-2 [1][2][3][4][5][6][7]. Several mechanisms could explain the ability of these oral pathogens to exacerbate lung infection including their oral inhalation into the lower respiratory tract, by swallowing contaminated oral fluid, but also by the oral localization of host receptor-proteases-mediated pathways facilitating their viral infectivity [5][8][9].
The transmembrane protein receptor ACE2 (angiotensin 2 converting enzyme), as well as TMPRSS2 (transmembrane serine 2 protease) and furin enzymes, have been identified as critical determinants of oral SARS infectivity [10]. ACE2 is expressed on different cells of oral tissues including oral mucosa, gingiva, tongue, salivary glands, and tonsils [11][12][13][14][15][16][17][18] (Figure 1). Almost 96% of ACE2-positive oral cells would locate in dorsal tongue. Epithelial cells of the oral cavity showed abundant expression of ACE2 receptor, that is also expressed in T cells, B cells, and fibroblasts, although to a lesser extent [12][16]. ACE2 is reported to be predominantly localized to the basal cells of stratified squamous epithelium but was also visible in the horny layer of keratinized epithelium and finally, in tongue coating [11][12][13]. Interestingly, gingival sulcular epithelium tended to display stronger ACE2 expression than the buccal gingival epithelium [13]. The presence of ACE2 is confirmed in the taste epithelial cells of tongue fungiform papillae. The epithelial cells of salivary ducts and serous cells of human submandibular glands express abundantly ACE2 [13][17][18]. Its expression in epithelial cells of minor salivary glands is even higher than in lung cells, and could constitute a reservoir zone for SARS-CoV-2 in asymptomatic patients [15][18]. Interestingly, TMPRSS2 and furin were found to be expressed globally in the same oral tissues as ACE2 (dorsal tongue, gingiva, salivary glands, taste buds) [13][18][19]. TMPRSS2 is expressed in the squamous epithelium of the tonsils [20][21]. Oral localization of furin was not systematically associated with that of TMPRSS2 and ACE2. Furin-positive cells were neither observed on the surface of the squamous epithelium of the dorsal tongue and salivary ducts, nor on tongue coating. Conversely, furin was secreted in saliva like TMPRSS2 [13]. TMPRSS2 may play a larger role in oral infection compared to furin, and ACE2–TMPRSS2 co-expression is a privileged target for SARS-CoV-2 infection.
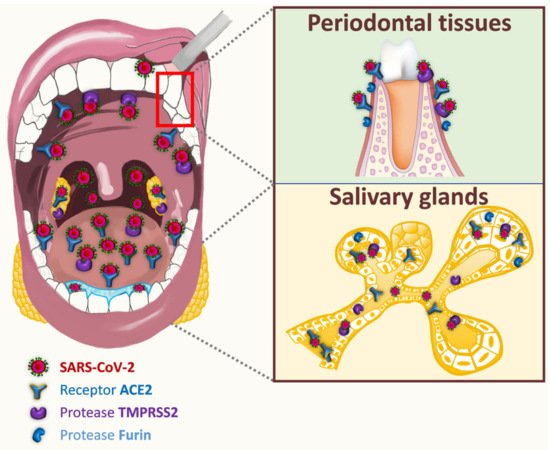
Figure 1. Potential entry routes for SARS-CoV-2.
The membrane protein neuropilin (NRP1) and extracellular MMP inducer (EMMPRIN) have been recently considered as other targets for SARS-CoV-2 infectivity. NRP1 is expressed in the differentiated epithelial cell layers of human normal tongue and in epithelial cells of human healthy salivary glands. The neuropilin-1 receptor is up-regulated in dysplastic epithelium and oral squamous cell carcinoma [22][23][24][25][26]. EMMPRIN expression is also up regulated in oral squamous cell carcinoma. Since ACE2 expression is depleted in oral squamous cell carcinoma, EMMPRIN receptor might be taken over for SARS-CoV-2 entry into cancer host cells [23][27]. The oral expression of all these factors indicate that oral cavity may be vulnerable to SARS-CoV-2 invasion.
While Wang et al. have reported a proliferation of SARS-CoV in exfoliated epithelial cells in saliva [5], SARS-CoV-2 is detected with a sensitivity of 89.8% on the surface of the tongue after swabbing [28]. To our knowledge, there is only one article demonstrating the direct presence of SARS-CoV-2 in COVID-19 autopsy oral tissues such as human salivary glands and mucosa. In particular, SARS-CoV-2 was detected in oral squamous keratinocytes [18].
Dysgeusia and xerostomia (early symptoms associated with SARS-CoV-2 infection) [17][29][30][31], but also some oral manifestations such as tongue ulcers [32], could be related to the presence of SARS-CoV-2 invasion factors (such as ACE2 and TMPRSS2) on the taste buds and dorsal tongue [18]. Interestingly, the expression of ACE2 and TMPPRSS2 in gingival sulcular epithelium (directly linked to gingivitis or periodontitis) [13], and the detection of SARS-CoV-2 in the inflammatory gingival crevicular fluid [33], raise questions on the possible role of this epithelium in SARS-CoV-2 infection. The potential passage of SARS-CoV-2 through the systemic route [34] could be considered as it has been demonstrated for periodontal bacteria such as Porphyromonas gingivalis [35]. It might be possible to imagine the risk of co-infection between SARS-CoV-2 and bacteria of the periodontal pocket. Co-infection of influenza virus and Porphyromonas gingivalis could initiate in vitro the autophagy of pulmonary epithelial cells [36].
Whole saliva is a biological fluid secreted by major and minor salivary glands and contains gingival crevicular fluid (GCF), desquamated oral epithelial cells, dental plaque, bacteria, nasal and bronchial secretions, blood and exogenous substances [37]. The detection of SARS-CoV-2 in saliva was first reported in 11 COVID-19 patients (91.7%) in Hong Kong [38]. Since then, more than 250 publications have revealed the presence of SARS-CoV-2 in saliva, in connection with the development of saliva diagnostic tests for COVID-19. At least four different pathways for SARS-CoV-2 entry are suggested into saliva: first, by major and minor salivary gland infection; second, from the lower and upper respiratory tract (sputum, oropharynx, cough); third, from the blood into the GCF and fourth, from dorsal tongue [28][39]. Since SARS-CoV has been shown to be able to infect epithelial cells in salivary gland ducts, as early as 48h after its intranasal inoculation in rhesus macaques [14], autopsy of human salivary glands from COVID-19 patients confirmed SARS-CoV-2 infection in these tissues [18]. Furthermore, SARS-CoV-2 nucleic acids were detected in pure saliva from mandibular salivary glands [17]. The salivary glands could constitute a direct source of the virions in the saliva. Saliva is principally secreted from the salivary glands but can contain secretions coming down from the nasopharynx or from the lung, especially later in infection. Saliva samples obtained by coughing up saliva from the posterior oropharynx, were collected from 23 SARS-CoV-2 infected patients. Of these, 87% were tested positive for SARS-CoV-2 [38]. Yet, it is possible that these samples included secretions from the nasopharynx or lower respiratory tract. A passive contamination of sputum could affect the kinetics of saliva [40][41]. Some SARS-CoV-2 positive ciliated cells originating from nasal cavity are found in the saliva [18]. SARS-CoV-2 infected GCF establishes the possible contribution of this fluid to the viral load of saliva [33]. Finally, the presence of SARS-CoV-2 on the dorsal tongue and in infected squamous epithelial cells in saliva [18][28] provides a potential cellular mechanism for spread and transmission of SARS-CoV-2 by saliva.
SARS-CoV-2 viral RNA load in oral fluid globally ranged from 9.9 × 102 to 7.1 × 1010 copies/mL [42][43][44][38][45][46][47][48][49]. The peak was globally reached during the first week of symptom onset and declined over time with gradual symptom improvement [42][43][4][38][45][46][47][48][50][51]. A high load in the pre-symptomatic phase could also be expected [52]. During the period of virus shedding, viral RNA could be detected up to 25 days after symptom onset [42][43][5][38][41] and in one case report, up to 37 days [53], independently of the severity of the illness [5]. Few studies have reported an association between viral loads and severe symptoms [43][38][50][54]. Although in a study using posterior oropharyngeal saliva, viral loads were found higher (1 log10 higher) in patients with severe disease compared to patients with mild disease, this relationship was not statistically significant [38]. No significant difference was observed in disease severity or clinical symptoms between patients in whose saliva viral RNA was detected or undetected [50]. However, the prevalence of severe disease and cough were frequently higher in patients in whom viral RNA from saliva was detected [40]. Interestingly, several studies have reported the presence of viral RNA in the saliva of asymptomatic patients [45][50][55][56][57]. Salivary SARS-CoV-2 RNA was detected in more than 50% of asymptomatic patients and of patients before the symptom onset [50]. Among 98 asymptomatic health-care workers, two individuals were tested negative for matching self-collected nasopharyngeal samples, but positive in saliva [42]. Alternatively, saliva samples from symptomatic patients with negative SARS-CoV-2 NPS could also be positive [58][59]. Saliva may be more sensitive in detecting asymptomatic or pre-symptomatic infections. The timing and duration of infectivity are important to establish, especially for asymptomatic individuals, because the risk of transmission by air through salivary droplets is possible. Indeed, the relationship between SARS-CoV-2 detection, viral load and infectivity is still unclear as viral RNA may not represent infectious transmissible virus. Viral culture studies using COVID-19 patients to confirm the presence of infectious SARS-CoV-2 are limited. A positive viral culture of infectious virus was found from the saliva of three patients [46]. The infectivity of SARS-CoV-2 in saliva has been demonstrated, even 15 days after the onset of clinical symptoms, using cell culture and an animal model [60]. A recent study suggested that no viable virus could be cultured from salivary swab specimens collected from COVID-19 patients with prolonged viral RNA shedding (>20 days after diagnosis) [61]. The risk of virus transmission can therefore be expected to be low, even though late viral shedding is present in asymptomatic or mildly symptomatic patients. Further investigations with larger cohorts and standardized procedures are necessary to precise the correlation between salivary viral loads, disease severity, infectivity of salivary virus.
SARS-CoV-2 is transmitted to human either by hand carriage or by airborne route. In both cases, the virus originates from nose and/or mouth of an infected patient when breathing, speaking, sneezing, coughing or during dental treatments. By breathing, the warm (36 °C) and moist (6.2% water) gases produced in alveoli rise to the mouth and nose where they cool and condense before being expelled (0.6 to 1.4 m/s) in the form of droplets by the respiratory flow. These droplets (0.8–1 µm diameter) contain water and mucous particles from the alveolar and the upper respiratory tract, and the eventual infectious agents. They form a bio-aerosol and can contaminate nearby people but can also remain in the atmosphere (Figure 2). The questions of virus viability duration and concentration in air remain unsolved [62]. Speaking differs by the vibrations of the vocal cords, the longer exhalation time, and the typical flow and pression due to some consonants. Thus, droplets are sprayed from 0.5 to 3 m with possible contamination (Figure 2). The same question of virus viability duration and concentration in the air remains [63]. By coughing and sneezing, air expulsion is brutal (up to 13 m/s), resulting in the transport of a large amount of alveolar, nasal/oral mucous materials and infectious agents included in very large droplets up to 100 µm [64]. In a few milliseconds, the droplets flatten and split up over a distance of 0.7 m. The heaviest particles fall down and contaminate the underlying surfaces which become fomites. In 10–20 s, the largest droplets lose water through evaporation, mostly in case of low relative humidity and high atmospheric temperature [65]. The resulting little particles with a low water content (i.e., droplet nuclei) and can stay in the atmosphere for many hours or even days (Figure 2). The aerial viral load can therefore increase over time, mostly in closed spaces without sufficient ventilation. Inhaled airborne viruses deposit directly into the human respiration tract. Finally, airborne transmission appears to be highly virulent and represents an important transmission route of the disease [66].
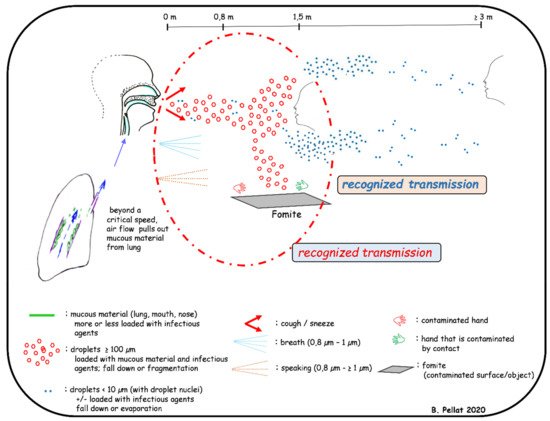
Figure 2. Aerosolization mechanisms of oral and nasal fluids.
Direct droplet and airborne transmissions of SARS-CoV-2 occur at variable distance and extended duration [67]. The droplets and droplet nuclei containing SARS-CoV-2 fall down (<1.5 m and several meters, respectively) and contaminate the surrounding surfaces which become fomites (Figure 2). Viral transmission from contaminated surfaces or fomites has a long history, including self-inoculation of the oral, nasal and ocular mucous membranes by hands that have touched these surfaces [68]. This transmission route is important in dental settings where aerosolization of droplets containing SARS-CoV-2 is also generated by many dental instruments. The bio-aerosols produced could be found several meters from the patient’s mouth and could remain in the atmosphere of the treatment room for several hours before settling on the worktops [62].
Regarding the stability of viruses on surfaces, the persistence of SARS-CoV-2 infectivity on fomites has been analyzed by spraying a solution containing the virus onto various surfaces [69]. The stability is higher on plastic and stainless-steel surfaces, 72 h and 48 h, respectively, than on copper and cardboard, 4 h and 24 h respectively. Another study showed that internal and external protective masks may be contaminated for several days with SARS-CoV-2 [70]. These results increase the probability of transmission by contact with fomites since the virus can remain viable several days on supports (plastic, steel) that frequently found in the medical environment [70][71].
Suspected COVID-19 patients are symptomatic patients showing signs of COVID-19 (see Q35) or asymptomatic patients in close contact—within the previous 14 days—with another person infected or presenting these symptoms [72]. Confirmed COVID-19 patients are symptomatic or asymptomatic patients who have been tested positive for SARS-CoV-2 with rRT-PCR or rapid antigen test [73]. The early and rapid recognition of infected patients and patients in close contact with COVID-19 infected individuals aims at limiting contacts with others to break the viral chains of transmission [72]. Screening questionnaire based on the criteria of confirmed/suspected SARS-CoV-2 infection should be carried out by telephone or by internet when a patient makes an appointment, and at the dental office entrance [74][75].
Patients should access the dental office only by appointment [74]. To minimize contact with other patients, only one single patient is ideally allowed in the waiting room with waiting time as short as possible [74][76]. The planning schedule should be set with sufficient time for patients’ appointments [74][75][77]. During the COVID-19 outbreak, patients should not be accompanied to the dental office unless necessary. Only essential persons such as parents of pediatric patients and guardian of patients presenting intellectual disability are allowed [74][75][76]. The presence of these persons is prohibited (if possible) during aerosol-generating procedures (AGPs) [76]. Patients should have their appointment be rescheduled if they show symptoms of COVID-19 within 10 days, if they have been tested positive for SARS-CoV-2 infection within 10 days, or if they have had close contact with a suspected/confirmed COVID-19 person within 14 days, prior to their scheduled appointment [77]. In case of dental emergency, their appointment must be set at the end of the day [75].
For patients who seem to be “negative” for COVID-19, all dental cares can be provided by applying the standard precautions and using a respirator for aerosol-generating procedures (AGPs). Patients with suspected/confirmed COVID-19 should not enter the dental facility, unless they need urgent dental care [74]. Only dental emergency should be handled minimally invasively—without AGPs if possible—in a well-ventilated room. The dental staff in the treatment room should be limited to essential personnel and the doors should always remain closed during treatment. Dental staff should apply standard, contact and droplet precautions when performing clinical exam, and add airborne precautions when performing AGPs (see Section 10.3 and Section 10.4) [72][75][76]. Tele-dentistry (i.e., telephone consultations or videoconferencing) could be an alternative to face-to-face outpatient visits, providing clinical support and pharmacological treatments without direct contact with suspected/confirmed COVID-19 patients [76]. An appointment can be made after the contagiousness period (see Q47 and Q57).
Early detection of SARS-CoV-2 infection among dental staff members may be achieved through daily self-assessment for signs and symptoms of COVID-19 [75][78], and laboratory testing in case of suspected SARS-CoV-2 contamination [78].
Dental staff members exposed to SARS-CoV-2—due to a close contact with a COVID-19 person without appropriate personal protective equipment—should be excluded from work, self-monitor their symptoms and self-quarantine for 14 days [77][79][80]. They should be tested [77][79]. A rRT-PCR test on day 10 after exposure can be performed and if it is negative, quarantine can be discontinued earlier [80]. Dental staff member presenting symptoms that are compatible with COVID-19 should stop working, self-isolate at home [74][75][77] and get tested [74][79]. A dental staff member with a positive SARS-CoV-2 test—with or without symptoms—should self-isolate at home. The safe return to work can be achieved after at least 10 days (minimum 20 days for severe COVID-19 and for immunocompromised staff member) with an additional 24 to 72h without fever associated with improvement of respiratory symptoms [78][81].
Standard precautions are designed to reduce the risk of pathogen transmission, including bloodborne and airborne pathogens. They include hand and respiratory hygiene, use of appropriate personal protective equipment based on the risk assessment [75] (see Section 10.5), care equipment and environmental cleaning, and safe waste management [82].
Hand hygiene is one of the most effective method to prevent pathogen transmission and healthcare-associated infections [82][83], including COVID-19 [77]. Dental staff members should apply WHO’s “My five moments for hand hygiene” approach: before touching a patient, before a clean or aseptic procedure, after body fluid exposure risk, after touching a patient, and after touching patient surroundings (whether or not gloves are worn). In addition, hand hygiene should be performed before putting on personal protective equipment and after removing them [77][82][83][84][85]. To perform hand hygiene, nails should be kept natural (without nail polish, artificial fingernails or extenders) and short (≤0.5 cm). Wearing watches, rings or other jewelry is discouraged, and long-sleeves should be avoided [84]. When hands are not visibly dirty or soiled, the preferred method is to use an alcohol-based hand rub for 20−30 s until they are dry [82][83][84]. Virucidal activity of hand rub agents is tested by EN 14476 (European Committee for Standardization standards) or by ASTM E1838 (American Society for Testing and Materials standards). When hands are visibly dirty or soiled with blood or other body fluids, hands must be washed with plain soap and water for 40−60 s [82][83][84].
Controlling the spread of pathogens from the source is key to avoiding any transmission. Standard respiratory hygiene precautions should be applied to every person exhibiting respiratory symptoms (coughing or sneezing) [82]. Respiratory hygiene precautions are taken during influenza and SARS-CoV epidemics. They are as follows: cover nose and mouth with a disposable/single-used tissue or bent elbow when coughing or sneezing, discard used tissues and masks, and perform hand hygiene after any contact with respiratory secretions or objects potentially contaminated with respiratory secretions [76][82][86]. During COVID-19 outbreak, patients and visitors should wear a medical or cloth mask in the dental facility to prevent the spread of respiratory secretions due to potential asymptomatic and pre-symptomatic transmission [75][77]. Patients should be provided with hand hygiene means, paper tissues and masks in common areas (i.e., reception area and waiting room) [75][76][77][82][87].
Additional precautions are supplementary infection prevention and control measures required by dental staff members to protect themselves and prevent transmission of pathogens like SARS-CoV-2 [87][88]. They include contact, droplet and airborne precautions [86]. During the COVID-19 outbreak, spatial distancing of at least 1–1.5 m should always be maintained between patients [74][75][76][77][87]. It should be also maintained between dental staff members when they need to be unmasked (when eating and drinking) [75]. It can be only broken by dental staff members during a patient’s dental treatment. In addition, use of physical barriers such as glass or plastic panels as protection against respiratory droplets can reduce dental staff members’ exposure to SARS-CoV-2, especially in the reception area [74][75][76][87]. It does not exempt patients and dental staff members from respecting spatial distancing and the use of masks [74].
SARS-CoV-2 is mainly transmitted through respiratory droplets (>5 µm in diameter) and contact routes (see Q32 and Q33). Droplet transmission occurs when a person is in close contact (within 1 m) of infected people. Their mucosae (mouth, nose, eyes) are therefore exposed to infectious respiratory droplets. Transmission can also occur through direct contact with infected people and indirect contact with surfaces (fomites) in the immediate environment or with medical devices previously used on an infected person [76]. Therefore, contact and droplet precautions should be implemented by dental staff caring for each suspected/confirmed COVID-19 patient [72]. They comprise the use of appropriate personal protective equipment (PPE): medical mask, eye protection, non-sterile long-sleeved gown, and medical gloves (see Section 10.5) [76][89]. PPE must fulfil quality standards (European Committee for Standardization [CEN] or American Society for Testing and Materials [ASTM] standards for instance) [78]. A new set of PPE is needed when providing care to a different patient. Dental staff members should refrain from touching their eyes, nose or mouth with potentially contaminated gloved or bare hands [76].
Airborne transmission refers to the presence of droplet nuclei (<5 μm in diameter) which can remain in the air for longer periods of time and can be transmitted to others for distances greater than 1m (see Q32). Airborne transmission of SARS-CoV-2 is possible in settings where aerosol-generating procedures (AGPs) are performed [76]. During the COVID-19 outbreak, airborne precautions should be applied by dental staff for each AGP [74] (e.g., use of high-speed dental turbine and handpiece, air/water syringe, ultrasonic scaler, air polishing, and air abrasion) [75]. They rely on the use of appropriate personal protective equipment: respirator, eye protection, non-sterile long-sleeved gown, and medical gloves. If gowns are not fluid resistant, dental staff members should use an additional water-resistant apron. In addition, the dental treatment room should be ventilated [76].
To relieve patients from isolation, negative rRT-PCR tests are not required [90]. Indeed, the detection of viral RNA does not necessarily mean that a person is contagious. The duration of rRT-PCR positivity generally appears to be 1-2 weeks for asymptomatic patients, and up to 3 weeks or more for symptomatic patients [72].
Criteria for releasing COVID-19 patients from isolation are:
For symptomatic patients: at least 10 days after symptoms onset (14 to 20 days for severe COVID-19, and 20 days for immunocompromised patients) with an additional 24 to 72 h without fever associated with improvement of respiratory symptoms.
For asymptomatic cases: 10 days after positive SARS-CoV-2 test [90][91][92].
Appropriate use of personal protective equipment aims to reduce, but not eliminate, the risks of transmission of respiratory pathogens to dental staff [86].
According to standard precautions, medical gloves are indicated in all clinical situations at risk of contact with blood, body fluids, secretions, excretions and items visibly soiled by body fluids, and in cases of contact with mucosae and non-intact skin of patients [83][84][93]. In addition, they are indicated for handling/cleaning instruments, handling waste and cleaning environmental surfaces in the dental facility [83][84]. Their use does not replace the need for proper hand hygiene [83][88]. It is recommended to change them between each patient, and to perform hand hygiene immediately after their removal [82]. Washing or decontaminating gloved hands is strictly prohibited [84][93]. The double gloving is not recommended for COVID-19 patients [87]. Gloves should be removed as soon as they are damaged (or non-integrity suspected). They should also be removed as soon as dental treatment has been completed, and when there is an indication for hand hygiene [80][84].
Masks are indicated for the protection of healthy people. Wearing a mask allows to protect oneself in case of contact with a COVID-19 patient, and prevents onward transmission of the virus when used by a COVID-19 patient [89].
For the general population, the cloth mask is recommended as an alternative to the medical mask during COVID-19 outbreak in public places where there is community transmission and where other prevention measures, such as physical distancing, are not possible [72][89]. Patients and visitors should wear their own cloth mask upon arrival and throughout their stay in the dental facility. Patients may remove them in the dental treatment room, but they must put it back on at the end of dental treatment [75]. For dental staff, the use of cloth masks as an alternative to medical masks is not considered appropriate [87][89] because cloth masks are not personal protective equipment [75]. In addition, cloth masks are not fluid-resistant and thus may retain moisture, become contaminated, and act as a potential source of infection [87].
Medical masks—also known as surgical masks—are indicated for dental staff member and at-risk individuals [89]. Continued use of a medical mask by dental staff members is recommended during all routine activities throughout the entire shift [72][75][77]. Dental staff members caring for COVID-19 patients without aerosol-generating procedures (AGPs) may wear a medical mask. Medical masks should be type IIR (EN 14683 [European Committee for Standardization standards] or tested by ASTM F2100 [American Society for Testing and Materials standards]) [89].
Particulate respirators—also known as filtering facepiece respirator—offer greater filtration capacity. Whereas medical masks filter 3 µm droplets, respirators filter out 0.075 µm solid particles [89]. Thus, medical masks do not offer adequate respiratory protection against aerosols (droplet nuclei), especially due to leaks around the edge of the mask when the user inhales [86]. Use of a respirator is required in dental treatment room where AGPs are performed, especially for COVID-19 patients [75][77][89][94]. In addition, according to ECDC and CDC, respirators are indicated when managing a suspected/confirmed COVID-19 patient (with or without AGPs) [75][77][94]. Respirators should be FFP2 or FFP3 (EN 149; European standards), N95 (NIOSH-42CFR84.181; US standards), or KN95 (GB 2626-2006; Chinese standard) [89]. Moreover, respirators with exhalation valves should not be used during surgical procedures as they allow unfiltered exhaled breath to escape [75][76].
To date, WHO, ECDC and CDC recommendations did not change regarding mask use despite the emergence of new SARS-CoV-2 variants, which have led to increased transmissibility [95][96][97]. However, some countries no longer accept cloth mask for the general population in certain places (e.g., hospitals, public transportation) and extend the use of respirators.
Correct use of mask/respirator consists in performing hand hygiene before putting on the mask, then placing the mask/respirator on carefully, ensuring it covers the mouth and nose, adjusting it to the nose bridge, and tying it securely to minimize any gaps between the face and the mask/respirator, and finally avoiding touching the mask/respirator while wearing it [89]. Regarding respirator, an initial fit testing is needed before use [76][94]. If the dental staff member has a beard, this may prevent proper fit of the respirator [76]. Mask/respirator should be removed if it is wet, soiled or damaged, if it is exposed to splashes, if it is touched or displaced from face for any reason [87][89]. The use of the same medical mask/respirator by a dental staff member between a confirmed/suspected COVID-19 patient and a patient who does not have COVID-19 is not recommended due to the risk of transmission [87]. Mask/respirator should be removed without touching their front, then a hand hygiene should be performed [89].
Medical mask and respirator are single-used personal protective equipment (PPE). They should ideally be changed after each patient [75][86]. However, during COVID-19 outbreak, which created severe shortages of PPE, medical masks and respirator could be used by dental staff without removing them for up to 6h and 4h, respectively [87][88]. However, wearing medical mask during a prolonged period increases the risk of contamination of the mask/respirator with SARS-CoV-2 and other pathogens. There is a risk that dental staff members will contaminate their hand by touching the front of the mask/respirator. If it is touched/adjusted, hand hygiene must be performed immediately [87]. The risk of contamination can be reduced by wearing a face shield over the mask [80]. Finally, wearing the same medical mask/respirator is only allowed to treat several patients who have the same COVID-19 status [80][88]. Methods of reprocessing medical mask/respirator—by disinfection or sterilization—are neither well established nor standardized. No evidence is available to date on the reprocessing of medical mask/respirator [87].
Eye protection—such as goggles and face shield—are indicated to reduce the risk of droplets transmission and splashes to the ocular mucosa [89][94]. Face shield covers and protects the entire face from splashes, including the side of the face and the chin [87]. Conventional eye glasses should not be used as eye protection [86]. During COVID-19 outbreak, dental staff should wear eye protection associated with their medical mask/respirator during all patient care [75]. Immediately after removal, goggles and face shield should be decontaminated, and hand hygiene should be performed [87].
According to the additional precautions, a long-sleeved water-resistant non-sterile gown is indicated to protect skin and prevent soiling of work clothes during treatment and activities that may generate splashes of blood or body fluids, and during aerosol-generating procedures (AGPs) [80][82][94]. When used, gowns should always be changed after each patient contact [80]. Immediately after removal, single-use gowns should be discarded and hand hygiene is required [82]. Cloth gowns can be decontaminated for reprocessing by machine washing them at high temperature (60–90 °C) and laundry detergent [87]. If gowns are not water-resistant, dental staff should use an additional disposable water-resistant apron over the gown [76][94]. Water-resistant plastic aprons should not be used alone when performing AGPs on COVID-19 patient [87].
Before dental cares, CDC and ECDC suggest the following sequence to put on personal protective equipment (PPE): (1) perform hand hygiene, (2) put on a clean gown or apron, (3) put on a medical mask/respirator, (4) put on eye protection, and (5) put on clean gloves [75][94]. After completion of dental cares, CDC suggests the following sequence to remove PPE: (1) remove gloves, (2) remove gown or apron, (3) perform hand hygiene, (4) remove eye protection, (5) remove and discard surgical mask/respirator, and (6) perform hand hygiene [75].
Procedures for cleaning and disinfecting the dental environment aim to reduce any role fomites may play in the transmission of SARS-CoV-2. The SARS-CoV-2 virus remained viable for up to a few days on surfaces, but it is an enveloped virus with a fragile outer lipid envelope that makes it sensitive to disinfectants [98]. Materials, objects, and devices should be stored in a way that facilitates environmental cleaning and disinfection [74]. In the waiting room, toys, magazines, books or other non-essential items that patients may touch should be removed [74][75]. All surfaces in dental facility should be regularly cleaned and disinfected, especially high-touch surfaces, and whenever they are visibly soiled or contaminated with body fluids [76][87]. In common areas, high-touch surfaces require regular cleaning at least twice a day. In dental treatment rooms, high-touch surfaces should be disinfected after each patient visit [74][98] and terminal cleaning is required for low-touch surfaces, high-touch surfaces and floors at least once a day [98].
After ventilation, surfaces should be thoroughly cleaned using a detergent-disinfectant product effective against viruses following the manufacturer’s instructions [74][75][99]. Virucidal activity of disinfectants is tested by EN 14476 (European Committee for Standardization standards) or by ASTM E1053 (American Society for Testing and Materials standards). Cleaning should progress systematically to avoid missing areas, from the least soiled (cleanest) to the most soiled (dirtiest), and from higher to lower levels [98]. Cleaners should wear adequate personal protective equipment: water-resistant apron (or a long-sleeves water-resistant gown after a suspected/infected COVID-19 patient), gloves, medical mask (or respirator in a room were aerosol-generating procedures have been performed) and eye protection [98][99].
No-touch disinfection technology, such as UV irradiation or vaporized hydrogen peroxide, can complement but not replace the first manual cleaning of environmental surfaces that are required to remove organic material [98]. The effectiveness of alternative disinfection methods (e.g., ultrasonic waves, UV irradiation, and blue LED light) against SARS-CoV-2 are not known [75].
Dental staff should perform routine cleaning, disinfection, and sterilization protocols of medical devices [75].
To decontaminate work clothes, machine wash at high temperature (60–90 °C) for at least 30 min and the use of laundry detergent is recommended [85]. If a hot-water cycle cannot be used, bleach or other laundry products for decontamination of textiles should be added to the wash cycle [99].
Healthcare waste generated during the care of suspected/confirmed COVID-19 patients are considered as infectious clinical waste and should be collected safely in clearly marked lined containers and sharp safe boxes [76][80][85][99]. Waste are disposed at least once a day [98]. Waste generated in the waiting room can be classified as non-hazardous and should be disposed of in sturdy black bags before being collected by municipal waste management services [85].
For suspected/confirmed COVID-19 patients, aerosol-generating procedures (AGPs) should be avoided as much as possible. When the AGP is required for dental treatment and cannot be postponed, the risk can be minimized by performing a preprocedural mouth rinse, applying rubber dam isolation, using evacuation aspirators/suction and practicing four-handed dentistry [74][75]. If an AGP was performed, the dental treatment room needs to be naturally or mechanically ventilated before admitting a new patient [74].
Adequate ventilation with fresh and clean outdoor air can play an important role to prevent the spread of airborne infections by reducing the concentration of infectious respiratory aerosols in indoor air. There are three methods for ventilating: natural (window), mechanical, and mixed-mode ventilation [76][86][100]. In dental treatment rooms, a minimum of 6 (ideally 12) air changes per hour is recommended by CDC and ECDC [74][75]. WHO recommends an average natural ventilation rate ≥ 60 L/s/patient or ≥ 12 air changes per hour for mechanical ventilation in an outpatient room with airborne precautions [100].
Air cleaners using a high-efficiency particulate air (HEPA) filter may be effective in reducing the concentrations of infectious aerosols for dental offices without adequate natural or mechanical ventilation [76][99][101]. However, the evidence for the effectiveness of HEPA filters in preventing coronavirus transmission is currently limited [76][80]. If used, the CDC recommends placing the HEPA unit near the dental chair—but not between a dental staff member and the patient’s mouth—and it should not draw air into or through the breathing zone of the dental staff [75].
Air cleaners using ultraviolet germicidal irradiation, air ionizers using negative ion and ozone generators have been proposed in addition to ventilation [75][78][100]. However, the evidence on their effectiveness is currently limited and they are potentially hazardous to human health [101].