1000/1000
Hot
Most Recent

CXCL16 is a chemotactic cytokine belonging to the α-chemokine subfamily. It plays a significant role in the progression of cancer, as well as the course of atherosclerosis, renal fibrosis, and non-alcoholic fatty liver disease (NAFLD).
A tumor mass comprises not just cancer cells but also tumor growth supporting cells and tumor-suppressing immune cells [1]. All these cells forming the tumor microenvironment secrete various factors into the intercellular space. To date, many of these factors have been characterized. The most significant of these are chemokines, a group of approximately 50 chemotactic cytokines [2]. Earlier studies have characterized chemokines as a significant factor in immune system cell function [3][4]. However, over time, more studies have emerged showing the significance of a variety of chemokines in cancer development and growth [5]. One such chemokine is CXC motif chemokine ligand 16 (CXCL16), belonging to the sub-family of α-chemokines. To date, there has not been a review summarizing current knowledge on CXCL16 and its receptor CXC motif chemokine receptor 6 (CXCR6) in a tumor.
CXCL16 is a chemokine distinct from other CXC chemokines. The CXCL16 gene is located on chromosome 17p13 [6], separate from other chemokine genes, and it has poor homology with the other chemokines [7]. There are two CXCL16 transcripts, 1.8 kb and 2.5 kb in length, formed by alternative splicing [8]. Both transcripts differ in the 3′-noncoding regions and the location of their expression. The 1.8 kb transcript is found mostly in the spleen, thymus, and testis. In contrast, the 2.5 kb transcript is found in the heart, kidney, liver, lung, peripheral blood leukocytes, pancreas, and prostate [8]. Alternative RNA splicing produces two other transcripts having 70 (CXCL16v1) and 121 (CXCL16v2) extra nucleotides, as demonstrated in dendritic cells [9]. In these additional sequences, a STOP codon is present. This results in a shorter protein having only the chemokine domain. The other domains typically found in CXCL16 are not present.
After translation, a hydrophobic signal peptide is cleaved at the N-terminus of the CXCL16 polypeptide [7]. The human CXCL16 protein is 254 aa long [6][7], while the murine CXCL16 is 246 aa long and is 44% similar to the human CXCL16 [8]. The newly synthesized CXCL16 for both species is the so-called membrane-bound form of CXCL16 (mCXCL16) [6]. The structure of this protein is very similar to transmembrane CX3CL1 [10] because it consists of a small (24–27 aa long) cytoplasmic tail with a YXPV motif [6]. This motif can be phosphorylated on tyrosine to provide an SH2-binding site. mCXCL16 also consists of a CXC chemokine domain and a transmembrane domain (Figure 1) [6]. Both of these domains are separated from each other by a spacer region, approximately 100 aa long, rich in serine, threonine, and proline. It is a site of heavy O-glycosylation which results in the formation of a mucin-like stalk [6][7], essential for chemokine domain presentation and, therefore, significant for mCXCL16 properties [11][12]. mCXCL16 has a molecular weight of 60 kDa.
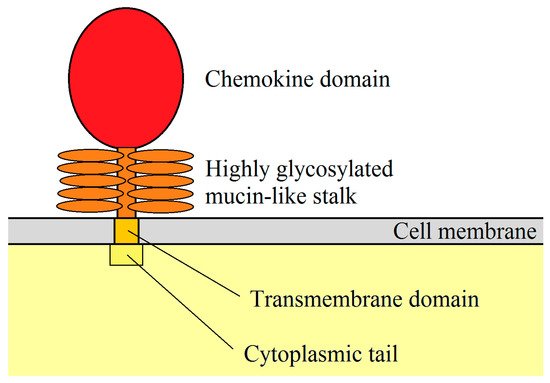
Figure 1. mCXCL16 structure. CXC motif chemokine ligand 16 (CXCL16) is a membrane protein consisting of a chemokine domain, highly glycosylated mucin-like stalk, transmembrane domain and cytoplasmic tail.
After CXCL16 cleavage, the 35 kDa chemokine domain is released and becomes a soluble form of CXCL16 (sCXCL16). This process is regulated by disintegrin and metalloproteinase 10 (ADAM10) [13][14][15]. However, disintegrin and metalloproteinase 17 (ADAM17) may also be responsible for sCXCL16 shedding in the absence of ADAM10 [16] or by using phorbol 12-myristate 13-acetate (PMA) as an inflammatory inducer [13]. Studies on mesangial cells have shown that pro-inflammatory cytokines increase sCXCL16 shedding via ADAM10 and ADAM17 [17]. Although sCXCL16 is released from mCXCL16, the remainder of the mCXCL16 (mucin-like stalk, transmembrane domain, and cytoplasmic tail) is still present in the cell membrane. These domains are firstly proteolytically cleaved by γ-secretases and then proteolytically degraded [15].
Importantly, many papers do not distinguish between two forms of CXCL16. Researchers just alter the expression of the CXCL16 gene or change the expression of two CXCL16 forms at the same time; then they test selected parameters of the experiment. In those cases, we report the findings as referring to the unspecified “CXCL16” which may denote both forms at the same time. When writing about mCXCL16 and sCXCL16, we mean the specific form of CXCL16.
CXCL16 is classified as an α sub-family chemokine because it has a CXC motif at the N-terminus [7]. Nevertheless, this chemokine lacks the ELR motif, which is common in other pro-angiogenic CXC chemokines [6][7]. CXCL16 is expressed in lymphoid organs such as the thymus, spleen, lymph nodes, and Peyer’s patches, but not in bone marrow. It is also expressed in the liver, lung, small intestine, and kidney [6][7]. Moreover, CXCL16 is highly expressed in the epidermis, where it is produced by keratinocytes [18]. mCXCL16 is expressed in macrophages [8][19] and is also present in splenic and lymph node dendritic cells (DC), blood myeloid DC, and monocyte-derived DC. Interestingly, its expression increases after DC maturation and after exposure to pro-inflammatory factors [6][19]. It has been also shown that CXCL16 is expressed on CD19+ B cells [7].
Both forms of CXCL16 have different functions. sCXCL16 is a chemokine that is responsible for the chemotaxis of cells bearing the CXCR6 receptor [6][7] while mCXCL16 is a transmembrane protein. mCXCL16 may have adhesion protein properties that bind to CXCR6 (Table 1) [12][20]. However, signal transduction from CXCR6 via mCXCL16→CXCR6 is not necessary for cell adhesion. This property of mCXCL16 is important in immune cell accumulation at an inflammation site due to increased mCXCL16 expression on vascular walls by pro-inflammatory cytokines [20]. mCXCL16 also mediates the adhesion of Gram-negative and Gram-positive bacteria [11]. This leads to bacterial phagocytosis by cells expressing mCXCL16, such as macrophages and DC [8][11]. mCXCL16 may also act as a receptor that causes signal transduction into the cell. This signal can be induced by sCXCL16 [21] as well as by CXCR6 [22]. In the first case, this effect is called inverse signaling; in the second case–reverse signaling. Additionally, signal transduction from mCXCL16 is insensitive to pertussis toxin [23], and so it has a different mechanism than signaling mediated by the G protein-coupled receptor (CXCR6). The sCXCL16-induced activation of mCXCL16 causes the activation of extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) [21][23] and Akt/protein kinase B (PKB) pathways [21]. This leads to increased proliferation and formation of apoptosis-resistant tumor cells [21][23]. In glioblastoma or melanoma cells, CXCR6 activates the mCXCL16→ERK MAPK pathway, which causes migration but not proliferation of tumor cells [22].
Table 1. Activities of the two different forms of CXCL16.
| Ligand | Receptor | Activated Signaling Pathways | Physiological Significance | References |
|---|---|---|---|---|
| sCXCL16 | CXCR6 | G protein-coupled receptor, PKB, ERK MAPK, calcium mobilization | Proliferation, migration, fibrosis, VEGF and CXCL8/IL-8 expression | [7][12][24][25][26][27][28][29][30][31][32][33] |
| sCXCL16 | mCXCL16 | insensitive to pertussis toxin, ERK MAPK, PKB | Proliferation, apoptosis resistance | [21][23] |
| CXCR6 | mCXCL16 | insensitive to pertussis toxin, ERK MAPK | Migration but not proliferation | [22][23] |
| mCXCL16 | CXCR6 | Cell adhesion | [12][20] |
CXCL16 expression is increased by pro-inflammatory cytokines. This is important for the accumulation of immune cells at the inflammatory reaction sites, but the influence of the various cytokines is cell-specific. In ectopic endometrial stromal cells, tumor necrosis factor α (TNF-α) increases CXCL16 expression [24]. In vascular smooth muscle cells, interferon-γ (IFN-γ) increases the expression of this chemokine, but TNF-α does not have such an effect [34]. In human umbilical vein endothelial cells (HUVEC), TNF-α, IFN-γ, interleukin (IL)-1β, and IL-6 increase the expression of CXCL16 [20]. Research on keratinocytes has shown that TNF-α, IFN-γ and peptidoglycan increase the expression of CXCL16 in these cells [18]. Also, the expression of CXCL16 is increased by ionizing radiation, which is important during radiotherapy [35][36]. Another factor that increases the expression of CXCL16 is hypoxia and hypoxia-inducible factor-1 (HIF-1) [37][38].
CXCL16 is involved in the pathogenesis of atherosclerosis. Inflammatory cytokines such as TNF-α, IFN-γ, IL-1β and IL-6 increase the expression of CXCL16 on endothelial cells, as shown in a study on HUVEC [20]. This leads to the adhesion of THP-1 monocytes, which are human monocytic leukaemia cells. A study on peripheral blood mononuclear cells (PBMC) shows that monocytes do not express CXCR6 [39], which means that in the pathogenesis of atherosclerosis, monocytes may not be directly recruited through the CXCL16→CXCR6 axis. In principle, this effect may be indirect by recruiting CXCR6+ T cells [40]. CXCL16 plays an important role in the later stages of atherosclerosis. A high expression of CXCL16 is present in macrophages in the intima of atherosclerotic lesions but not in normal aortas [41]. mCXCL16 has scavenger receptor activity and can be a receptor for oxidized lipoproteins. For this reason, it was first named the scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX) [41]. As such, it can participate in the uptake of oxidized low-density lipoprotein (oxLDL) by macrophages [41] and vascular smooth muscle cells [34]. CXCL16 can also cause proliferation of aortic smooth muscle cells and increase the expression of TNF-α in these cells [26], which is associated with atherosclerotic vascular disease. A study in low-density lipoprotein (LDL) receptor-deficient mice showed that CXCL16 activity is athero-protective [42]. For this reason, more research is needed on the role of CXCL16 in the pathogenesis of atherosclerosis.
Another situation where CXCL16 plays a significant role is in liver diseases, in particular, nonalcoholic fatty liver disease (NAFLD). During inflammatory reactions, the expressions of CXCL16, CXCR6, and ADAM10 increase in the liver [43]. In NAFLD, hepatocytes produce CXCL16 [44] which activates hepatic stellate cells that produce collagen and transform into myofibroblasts [44]. Interestingly, activation of CXCR6 on hepatocytes reduces inflammation, fibrosis, and the death of these cells, as demonstrated in mice with an activated NF-κB pathway [45]. Despite this, CXCL16 increases lipid accumulation, extracellular matrix (ECM) excretion and reactive oxygen species (ROS) production in hepatocytes [43]. CXCL16 is also responsible for the accumulation of NKT cells in the liver [45][46][47][48]. These cells participate in inflammatory reactions, leading to liver fibrosis progression. Also, during septic shock, the expression of CXCL16 is increased in the liver [49]. This leads to the recruitment of activated T cells and consequently to endotoxin-induced lethal liver injury. Importantly, the NK and NKT cells are not responsible for septic shock symptoms in the liver.
Transplantology is another issue where CXCL16 plays an important role. During allogeneic heart transplantation in mice, the CXCL16→CXCR6 axis is involved in the recruitment of NKT cells into the transplanted organ [50], which is related to allograft tolerance. Nevertheless, CXCL16 can also cause graft-vs-host disease (GvHD) due to its participation in the recruitment of activated CD8+ T cells into the liver, leading to GvHD-induced hepatitis [51].
The CXCL16→CXCR6 axis is also involved in fibrosis in various organs. For example, during inflammatory reactions, the expression of CXCL16 increases on tubular epithelial cells [52]. This leads to the recruitment of bone marrow-derived fibroblast precursors, cells expressing CXCR6 [52][53], which participate in the pathogenesis of renal fibrosis. CXCL16 is also engaged in pulmonary fibrosis [30], which was demonstrated in human pulmonary fibroblasts (MRC-5 cell line), where this chemokine caused an increase in proliferation and collagen production. This was associated with the activation of the forkhead box O3a (FOXO3a) via the CXCR6→Akt/PKB pathway [30].
CXCL16 is also essential in the homeostasis of intestinal defense. It is responsible for the distribution of intestinal group 3 innate lymphoid cell (ILC3) subsets and IL-22 production [54]. This leads to an increase in antimicrobial peptide expression, which is involved in the defense against bacteria. CXCL16 can also be taken as a marker of inflammatory bowel disease (IBD) [55]. It seems that CXCR6+CD4+ T cells do not show colitogenic properties, in contrast to CXCR6−CD4+ T cells [56]. However, in inflamed colonic tissues, CXCL16 expression occurs on macrophages where it participates in the Th1 immune response [55].
The CXCL16→CXCR6 axis also participates in the development of endometriosis [24]. In ectopic endometrial stromal cells, TNF-α increases the expression of CXCL16. This causes migration and invasion of these cells, which is associated with the development of endometriosis.
CXCR6 is also an entry cofactor for human immunodeficiency virus (HIV)-1 [6][57][58][59] and HIV-2 [60] and so may be significant in the course of HIV infection. This is also supported by the association of polymorphisms in the CXCR6 gene with the control of viraemic disease [61][62]—its expression is downregulated in this disease [63]. In particular, the presence of specific polymorphisms in CXCR6 leads to rapid progression of acquired immunodeficiency syndrome (AIDS) [64] and influences the effectiveness of highly active antiretroviral therapy (HAART) [65]. Also, CXCL16 levels increase in HIV infection as the virus increases the release of this chemokine by macrophages [66]. This is associated with disease progression as CXCL16 increases HIV replication.