1000/1000
Hot
Most Recent

Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gut that includes ulcerative colitis (UC) and Crohn’s disease (CD). with colitis-associated colorectal cancer (CAC) being a progressive intestinal inflammation due to inflammatory bowel disease (IBD). While this is an exemplification of the negatives of inflammation, it is just as crucial to have some degree of the inflammatory process to maintain a healthy immune system. A pivotal component in the maintenance of such intestinal homeostasis is the innate immunity component, inflammasomes. Inflammasomes are large, cytosolic protein complexes formed following stimulation of microbial and stress signals that lead to the expression of pro-inflammatory cytokines. The NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome has been extensively studied in part due to its strong association with colitis and CAC. The aryl hydrocarbon receptor (AhR) has recently been acknowledged for its connection to the immune system aside from its role as an environmental sensor. AhR has been described to play a role in the inhibition of the NLRP3 inflammasome activation pathway.
The NLRP3 inflammasome is one of the most extensively studied NLRs due to its clinical relevance in a wide range of human diseases. This 115 kDa cytosolic protein complex consists of a triadic constitution; the NACHT scaffold which serves as a central oligomerization domain with an ATPase activity, the N-terminal PYCARD adaptor which recruits the apoptosis-associated speck-like protein (ASC) and the C-terminal leucine-rich repeat (LRR) which are thought to be involved in detecting stimuli.
While post-translational modifications play an important role in the regulation of the NLRP3 inflammasome activation, it can also be regulated through modifications at the transcription level with NF-κB. As previously mentioned, NF-κB plays a central role in the priming step and leads to the formation of pro-IL-1β, pro-IL-18 and the NLRP3 protein; which is needed for the activation of the NLRP3 inflammasome, following stimulation of the TLRs by Signal 1 [1][2]. Afonina et al. have identified several negative regulators of the NF-κB pathway that inhibit NLRP3 inflammasome activation, which may be the potential therapeutic target for inflammatory and autoimmune diseases [3].
Besides NF-κB, AhR has been shown to play a part in the innate immune response; such as in Listeria monocytogenes (LM) infections, where Kimura et al. revealed that AhR knockout macrophages had increased expression of the pro-inflammatory cytokines, IL-6 and TNF-α, and promoted caspase-3 activation; which lead to decreased macrophage survival and increased susceptibility to listeriosis [4]. The study further described that AhR also induces the expression of p40phox and the production of ROS, which further enhanced bacterial clearance during LM infection [4]. AhR and the NLRP3 inflammasome has been implicated in a study by Jia et al., which determined that their link was associated with the pathogenesis of acute myeloid leukaemia and an imbalance in the TH population; through measuring AhR and NLRP3 molecules in the bone marrow and peripheral mononuclear cells of newly diagnosed and remission patients [5]. Importantly, the myeloid-specific microRNA, miR-223 was identified as a negative regulator of the NLRP3 inflammasome through transcriptional control at NLRP3 3′-UTR [6][7]. Coincidentally, Ogando et al. determined that miR-223 acts as a regulator of the AhR/ARNT pathway and its expression leads to the down-regulation of the AhR/ARNT and increased pro-inflammatory cytokine production in rheumatoid arthritis patients [8]. Interestingly, miR-223 is a common regulator of both the AhR/ARNT and NLRP3 activation pathways.
The link between AhR and NF-κB was identified by Tian et al. who described that AhR and NF-κB RelA directly interact with each other by physical association and functional modulation following dissociation from their respective regulatory mechanisms after stimulation by their respective activation signals [9]. The study also proposes two mechanism models that may explain their mutual repression following their interaction; which are, the formation of an inactive complex or regulation by the transcription mediator p300/CREB-binding protein (CBP) that leads to competitive binding between ligand-AhR/ARNT complexes and RelA for p300/CBP [9]. This notion is supported by another study that determined NF-κB and the glucocorticoid receptor mutually repressed each other through the co-activators, CBP and steroid receptor coactivator-1 (SRC-1) [10]. In addition to NF-κB RelA, NF-κB RelB was also found to crosstalk with AhR [11][12]. Another link between AhR and the NF-κB pathway was demonstrated by Wang et al., in which they exemplified that quaking plays a role in inhibiting innate immune responses via regulation of the AhR/STAT1–NF-κB pathway [13].
The interactions between AhR and NF-κB lead to the hypothesis that AhR can also regulate NLRP3 inflammasome activity via the NF-κB pathway (Figure 4). A critical study by Huai et al. determined that AhR negatively regulates NLRP3 inflammasome activation at the transcriptional level via the NF-κB pathway by using TCDD-treated, LPS-induced mouse peritoneal macrophages to show reduced Nlrp3 expression at the protein and mRNA level [14]. They also identified that the inhibitory action was due to AhR binding to XRE sequences at the two NF-κB binding sites located in the NLRP3 promoter region [14]. Their proposed mechanism models were that NLRP3 acted as the rate-limiting element in its activation and that either AhR competed with NF-κB for binding sites or that AhR ‘tethered’ NF-κB to prevent the transcriptional process [14]. AhR has also been shown to negatively regulate LPS-induced inflammatory responses through its interactions with the NF-κB pathway via Stat1 to inhibit IL-6 activities [15] and via plasminogen activator inhibitor-2 (Pai-2) to inhibit IL-1β expression [16]. In addition to that, Zhu et al. also illustrated that the NF-κB pathway mediated LPS-induced AhR expression in macrophages [17]. However, it should be noted that a controversial study found that indoxyl sulfate-induced the expression of macrophage IL-1β via AhR-NF-κB/MAPK cascade, while bypassing NLRP3 activation [18]. This result may be specific to sustained chronic inflammation in the kidneys and may utilise different mechanisms than that of chronic colitis.
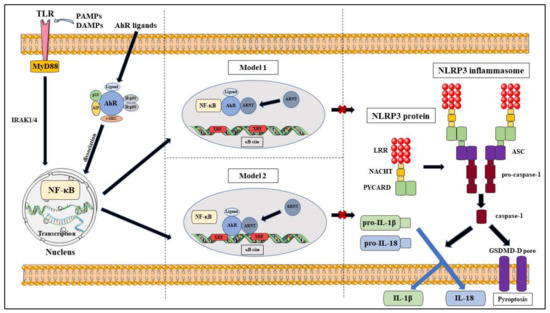
Figure 4. Proposed models of NLRP3 inflammasome inhibition via AhR pathway.
AhR and its effects on the NLRP3 inflammasome and colitis have been predominantly studied using wild-type (AhR+/+), AhR knockout (AhR−/−) and heterozygous (AhR−/+) mouse models that have been chemically induced with intestinal inflammation; by using 2,4,6-trinitrobenzene sulphonic acid (TNBS) or dextran sulphate sodium (DSS), and CAC; by using azoxymethane (AOM or AOM/DSS) [19]. Several studies have determined that AhR exhibits protective effects during intestinal inflammation; with TCDD-treated and FICZ-treated mice ameliorating colitis severity, which were determined from corrected weight loss, reduced colitis symptoms and recover periods [20][21][22][23][24]. These reversed colitis effects were due to a change in their cytokine environment, with AhR-activated mice having a reduction in the expression of pro-inflammatory mediators; such as IL-6, IL-12, IL-17, IFN-c, MCP-1, exotaxin-1 and TNF-α, but an increase in IL-22 and TGF-β [20][23][24]. The results from Monteleone et al. supports this notion with FICZ-treated TNBS-induced mice showing reversed colitis symptoms while also showing that colitis symptoms were enhanced when the mice were treated with AhR antagonists [25]. They further determined a link between FICZ and IL-22; with FICZ increasing the expression of IL-22, and anti-IL-22 leading to a reduction in the anti-inflammatory effects of FICZ [25]. Takamura et al. proposed that the inhibition of colitis by AhR may be due to the production of PGE2; and that the inhibition of PGE2 reduced the inhibitory effects of AhR on colitis [26]. Furumatsu et al. found that AhR knockout mice displayed more severe DSS-induced colitis when compared to the C57BL/6J wild-type mice [27]. In accordance to that, Arsenescu et al. proclaimed that wild-type mice developed severe colitis and AhR knockout mice died early in inflammation; but heterozygous mice exhibited little change in intestinal histology and had good clinical outcomes [28]. These heterozygous mice had reduced expression of TNF-α as compared to the other two models [28]. The disparity of results seen in these mouse models suggests more investigations to be done in order to achieve a greater insight into the mechanisms involved in the protective roles of AhR in maintaining intestinal homeostasis.
The understanding of the signalling pathways of AhR and the NLRP3 inflammasome and how they intertwine with each other is important in assessing the therapeutic potential of AhR in the treatment of IBD and CAC (Table 1). The abundance of natural AhR ligands found in the environment provides a positive outlook for future therapeutic agents that utilise AhR as an immunomodulator through its inhibitory effects on NLRP3 inflammasome activation. We have identified some NLRP3 inhibitors that utilise the AhR pathway have been described in an experimental context and have been demonstrated to be effective.
Table 1. Roles of the NLRP3 inflammasome and AhR in inflammation and cancer.
| Pathways | Mechanism | Ref. |
|---|---|---|
| AhR/STAT1–NF-κB axis | Quaking inhibits NF-κB transcriptional activity and prevents NLRP3 inflammation via AhR-dependent manner | [13] |
| NAD+/SIRT1/SUV39H1/H3K9me3 axis | Ameliorates colitis through promotion of Treg differentiation | [29] |
| LncRNA-PVT1-STAT3 axis | Inhibits STAT3 via LncRNA-PVT1 from promoting signals for cancer inflammation | [30] |
| AhR/Nrf2/NQO1 axis | AhR up-regulates Nrf2 and NQO1 levels in the colon and prevents NLRP3 inflammasome activation | [31] |
| Fibronectin/integrin β1/FAK axis | Promotes cancer metastasis through ANRT down-regulation | [32] |
Norisoboldine (NOR) is the primary isoquinoline alkaloid constituent of Radix Linderae; the dry root of Lindera aggregata (Sims) Kosterm (L. strychnifolia Vill), which is commonly used in Chinese medicine in treating a variety of ailments, which include constipation, polyuria, dyspepsia, acute and chronic colitis, chest and abdominal pain and rheumatism palsy [33][34]. NOR has been shown to be a natural AhR ligand by Qi et al. through its up-regulation of CYP1A1 expression, promotion of AhR nuclear translocation after dissociation of the AhR/Hsp90 complex and transcriptional activity of AhR/ARNT via DNA binding to the XRE region in THP-1 cells [34]. Studies also reveal that NOR promotes Treg cell differentiation from naïve T-cells in-vitro and increased its immunosuppressive effects on Teff cells through inducing apoptotic events and inhibiting Th1 and Th17 differentiation [29][35]. In the context of IBD, NOR has also been demonstrated to reduce NLRP3, ASC and caspase-1 expression in TNBS-induced mice, which could imply that NOR has inhibitory effects on NLRP3 inflammasome activation through an AhR pathway [34]. The study further found a link between NOR and Nrf2, a nuclear transcriptional factor of Cap’n’ Collar family that can inhibit NLRP3 priming [36], as the mechanism for NOR-induced inhibition of NLRP3 inflammasome activation; where NOR up-regulated the expression of Nrf2 but down-regulated the production of ROS signals in THP-1 cells [34]. Concurrently, Qi et al. also demonstrated that NOR was able to produce anti-UC effects through the suppression of glycolysis and promotion of Treg cell differentiation in hypoxic microenvironments via regulation of the NAD+/SIRT1/SUV39H1/H3K9me3 signalling pathway [29].
Cardamonin (20,40-dihydroxy-60-methoxychalcone) is a chalcone that is the main flavonoid isolated from the dry, mature seeds of the medicinal herb, Alpinia katsumadai Hayata [30][37][31]. It has been used for thousands of years as a traditional Chinese medicine due to its anti-inflammatory and anti-tumour properties [30][37][31]. The anti-inflammatory activity of cardamonin was studied by Wang et al., in which they demonstrated that cardamonin inhibited NLRP3 inflammasome expression and activation in mice colon with TNBS- and DSS-induced colitis and LPS- and ATP-stimulated macrophages [31]. They further displayed that AhR and Nrf2 were also influenced by cardamonin as evidenced by the up-regulation of CYP1A1 mRNA and protein levels, and the up-regulation of Nrf2 protein levels in THP-1 cells, respectively [31]. The AhR/Nrf2 signals produced by cardamonin would then activate NQO1, which has inhibitory effects in NLRP3 inflammasome priming, which lead to a reduction in pro-inflammatory cytokines and produce anti-colitis effects [36][31]. Further studies by Wang et al. revealed that cardamonin acts a broad-spectrum and specific inhibitor of the NLRP3 inflammasome through their mouse models which show that cardamonin increased the survival rate of mice with LPS-induced septic shock and down-regulated expression of TNF-α and IL-1β [37].
An early study discovered the link between AhR and colon neoplasia when they characterised the association of AhR with the human colon adenocarcinoma cell line LS180 [38]. This link was also observed in a study that utilised mouse models with sporadic colon cancer that were fed a Western-style diet; in which oxidative stress response and immune dysregulation via Nrf2 and AhR pathways were induced [39]. Another study using AOM/DSS-induced mouse models found that the absence of AhR increased their susceptibility in colorectal tumorigenesis. The same study also found that the AhR ligand, I3C protected against the development of colorectal tumours and is AhR-dependent [40]. The association between the NLRP3 inflammasome and AhR in CAC was shown in a study by Ikuta et al., in which cecal tumours developed in AhR-null mice in an ASC-dependent manner. This same study also determined that AhR plays dual tumour suppressor roles; as a regulator of intestine anti-inflammation and β-catenin degradation [41]. Cardamonin was also shown to have protective effects against tumorigenesis via AhR. The anti-tumour activity of cardamonin was explored by Wang et al., which indicated that cardamonin exerts anti-gastric cancer properties by down-regulating signal transducer and activator of transcription 3 (STAT3) via the LncRNA-PVT1-STAT3 signalling pathway in human gastric cells, AGS [30].
While NOR and cardamonin have been described to naturally inhibit NLRP3 activation and reducing the inflammatory effects via AhR, more studies should be conducted to expand the variety of these natural inhibitors.