1000/1000
Hot
Most Recent

Fibrinolytic factors like plasminogen, tissue-type plasminogen activator (tPA), and urokinase plasminogen activator (uPA) dissolve clots. Though mere extracellular-matrix-degrading enzymes, fibrinolytic factors interfere with many processes during primary cancer growth and metastasis contributing to the hallmarks of cancer. Their many receptors give them access to cellular functions that tumor cells have widely exploited to promote tumor cell survival, growth, and metastatic abilities. Fibrinolytic factors give cancer cells tools to ensure their own survival by interfering with the signaling pathways involved in senescence, anoikis, and autophagy. Dependent on the cell type, plasminogen was shown to either enhance or suppress cancer cell survival via autophagy. The Kringle 5 of plasminogen after engagement with the glucose-regulated protein 78 induces autophagy in endothelial cells.
Plasmin drives cellular extracellular matrix loss that induces programmed cell death or anoikis. Tumor cells by upregulating plasminogen activator inhibitor-1 suppress plasmin production. Now, they can escape anoikis and metastasize.
The preparation of the premetastatic niche is prepared by exosomes, small membrane-bound extracellular vesicles. Exosomes carry fibrinolytic factors and thereby might be important for ECM preparation during the metastatic process. Finally, fibrinolytic factors and their receptors including uPAR and LRP1 are upregulated in cancer cells after myelosuppressive treatment and contribute to drug resistance. Targeting receptors of fibrinolytic factors including uPAR and LRP1 can be a strategy to overcome drug resistance.
Proteolysis is required during normal development, as well as in pathological conditions, such as cancer and inflammation. Enzymes of the fibrinolytic cascade and their inhibitors contribute to carcinogenesis, cancer growth progression, and metastasis through a fine-tuned activation and deactivation of the contributing enzymes. Our understanding of fibrinolytic factors as simple fibrin and extracellular matrix (ECM) molecules that degrade enzymes has shifted. Fibrinolytic factors are also post-translational regulators of biomolecules, including chemo-/cytokines and cell surface receptors, and control the activity status of fellow proteases, such as matrix metalloproteinases (MMPs), ultimately establishing a proteolytic niche [1].
Fibrinolysis is the process of blood clot dissolution following hemostasis and clot retraction, which is an operation that keeps the blood flowing in vessels. The best-known fibrinolytic factor is the serine protease plasmin, which degrades fibrin (a major component of the clot) and degrades the basement membrane. Plasmin activity is controlled by alpha2-antiplasmin and alpha2-macroglobulin in the circulation. The plasminogen protein is an inactive zymogen that circulates in the blood plasma and is converted to the active protease plasmin by tissue-type plasminogen activator (tPA) and urokinase plasminogen activator (uPA). Plasmin activation also occurs after kallikrein and factor XII (Hageman factor) exposure, although with lower efficacies. The activity of uPA and tPA is inhibited by plasminogen activator inhibitor-1 and -2 (PAI-1 and PAI-2). PAI-1 can be produced by cancer cells [2], but also cancer-niche-associated cells, such as endothelial cells and activated platelets. An activated endothelium (due to thrombin, histamine, and bradykinin) and neurons or microglia release tPA. uPA is expressed on the bronchial epithelium, but also on some blood cells, such as monocytes, and in many cancer cells (Figure 1).
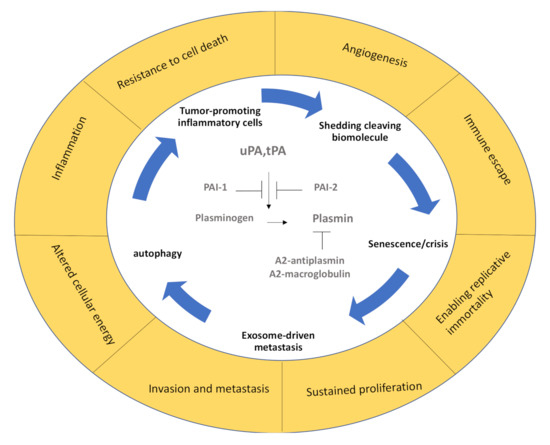
Figure 1. The fibrinolytic spiral during cancer. In the center, the main fibrinolytic factors and their endogenous inhibitors are depicted, which are surrounded by the pathogenic mechanisms they support. The outer circle summarizes the main hallmarks of cancer described by Hannahan and Weinberg [3]. PAI-1 and PAI-2: plasminogen activator inhibitor-1 and -2, tPA: tissue-type plasminogen activator, uPA: urokinase plasminogen activator.
Cancer cells take advantage of the pericellular plasmin generation on cell surfaces. Depending on the stage of the disease and the receptors involved, fibrinolytic factors like plasmin(ogen), plasminogen activators (PAs), and their receptors promote or suppress cancer progression or inflammation (recently reviewed in [4]). uPA can bind to uPA receptor (uPAR), which is a glycoprotein that is linked to the plasma membrane by a glycosylphosphatidylinositol anchor. It is composed of three domains (D1–3). The general structure and function of uPAR have recently been summarized [5]. Other PA receptors include a-enolase, low-density lipoprotein receptor-related protein (LRP1), epidermal growth factor receptor (EGFR), annexin II, and certain integrins. Through their ability to interact with other membrane receptors, PA receptors influence intracellular signaling activation of tumor-associated pathways.
Fibrinolytic factors control the critical hallmarks of cancer [3] (Figure 1). p53 is an important tumor suppressor that acts to restrict proliferation in response to DNA damage or the deregulation of mitogenic oncogenes by leading to the induction of various cell cycle checkpoints, apoptosis, or cellular senescence. Recent studies indicate that PAs and plasminogen receptors are p53 targets that accelerate primary tumor growth/metastasis and modulate anoikis (from the Greek word for “homelessness”) and senescence. Normal but not malignant cells undergo an apoptotic process termed “anoikis” after they lose contact with ECM or neighboring cells. Fibrinolytic factors, such as PAI-1 and PAI-2, can also be found in the senescence-associated secretory phenotype (SASP) complexes in cells that have reached a certain lifespan, promoting their entrance into the nondividing state of cellular senescence that can promote tumor growth.
Fibrinolytic factors also find their way into exosomes, which are secreted vesicles that harbor biomolecules, such as RNA, DNA, and glycans surrounded by a lipid bilayer (recently reviewed by [6]), and are known to enhance metastasis by establishing a prometastatic niche. From anoikis to senescence to autophagy and exosome-mediated metastasis promotion, along with other hallmarks of cancer [3], fibrinolytic factors modulate cancer progression. Below we review some recent studies in these exciting fields.
Autophagy produces nutrients and energy to enhance cell survival through the breakdown of cytosolic components within the autophagosomes. Cancers use autophagy-mediated recycling (including the degradation of apoptotic mediators) to meet the metabolic demand for constant growth, proliferation, and cell survival. The native plasminogen is a single-chain glycoprotein, which contains the N-terminal peptide, five homologous Krinkle domains (K1–5), and the protease domain. Tumor-suppressive functions have been reported for individual domains or larger domain fragments (reviewed elsewhere [7]).
Treatment with the plasmin inhibitor tranexamic acid resulted in the formation of autophagosomes in B16-F1 cells with positive cellular staining for microtubule-associated proteins 1A/1B light chain 3B (LC3), which is the most widely used marker of the autophagosome membrane [7]. Interestingly, the plasminogen/plasmin effects on autophagy in cancer cells seem to depend on the cancer cell type. Tykhomyrov et al. showed that plasminogen-treated lung cancer cells upregulated beclin-1 (Atg6), which is important for the generation of the isolation membrane that engulfs cytoplasmic material to form the autophagosome, and caused cell detachment in cancer, but not normal cells (Figure 2). The prosurvival effects of plasminogen-boosted autophagy occurred after treatment with the full-length plasminogen, but not the Kringle 1-3 (K1–3) fragments of plasminogen [8].
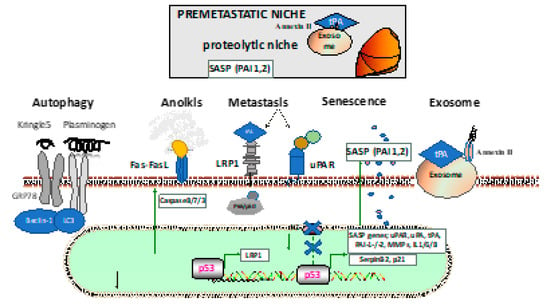
Figure 2. Establishment and interactions in the (pre)metastatic niche. The lower portion describes the mechanism through which fibrinolytic factors are involved in the cancer cells: autophagy through the Kringle 5 fragment or plasminogen; anoikis due to cell detachment with activation of the extrinsic apoptotic pathway through plasmin cleavage of the Fas ligand by plasmin; metastasis through pericellular plasmin activation via low-density lipoprotein receptor-related protein (LRP1) or uPA receptor (uPAR), alone or in complex with other membrane molecules (such as integrins or galectins); the participation of the senescence-associated secretory phenotype (SASP), including fibrinolytic factors like PAI-1 or PAI-2; the generation of fibrinolytic factor carrying exosomes that ensure the priming of the premetastatic niche in distant organs (such as the lung) with the establishment of the proteolytic niche. GRP78: glucose-regulated protein 78, LC3: microtubule-associated proteins 1A/1B light chain 3B, MMP: matrix metalloproteinase, PAI-1: plasminogen activator inhibitor-1, PAI-2: plasminogen activator inhibitor-2.
The glucose-regulated protein 78 (GRP78) is an endoplasmic reticulum (ER)-resident chaperone that binds polypeptide chains noncovalently on the ER and then dissociates, facilitating proper protein folding and assembly and helping protein transport across the ER membrane. GRP78 plays an important part in maintaining protein stability, regulating protein folding, and inducing apoptosis and autophagy. Kringle 5 (K5) of the human plasminogen can bind to the 78 kDa GRP78 [8]. This protein is activated by caspases and evokes an autophagic response in endothelial cells. Endothelial cells exposed to K5 up-regulated beclin-1 levels and progressively increased the amount of antiapoptotic Bcl-2 complexed with beclin-1. Prolonged exposure to K5 ultimately led to apoptosis via mitochondrial membrane depolarization and caspase activation in endothelial cells. Knocking down beclin-1 levels decreased K5-induced autophagy but accelerated K5-induced apoptosis [8]. It seems that plasminogen-mediated survival signals depend on the cell type and certain parts of the plasminogen protein.
Fang et al. showed that K5 decreased the expression of GRP78 via the downregulation of phosphorylated ERK, leading to caspase-7 cleavage and tumor cell apoptosis [9]. Furthermore, K5 promoted the sumo/ubiquitin-mediated proteasomal degradation of hypoxia-inducible factor 1 alpha by upregulating von Hippel–Lindau protein, resulting in the reduction of vascular endothelial growth factor and thus suppression of tumor angiogenesis.