1000/1000
Hot
Most Recent

5-aminolevulinic acid (5-ALA) is a porphyrin precursor in the heme synthesis pathway. When supplied exogenously, certain cancers consume 5-ALA and convert it to the fluorogenic metabolite protoporphyrin IX (PpIX), causing tumor-specific tissue fluorescence. Preoperative administration of 5-ALA is used to aid neurosurgical resection of high-grade gliomas such as glioblastoma, allowing for increased extent of resection and progression free survival for these patients. Targeting the heme synthesis pathway and understanding its dysregulation in malignant tissues could aid the development of adjunct therapies to increase intraoperative fluorescence after 5-ALA treatment
Glioma is the most common primary malignancy of the central nervous system and is classified as grades I–IV by the World Health Organization (WHO). In general, grade I and II gliomas are considered “low-grade” (LGG) and comprise astrocytoma (pilocytic and diffuse subtypes) and oligodendrogliomas, while grade III and IV lesions, including anaplastic astrocytoma and glioblastoma (GBM), are considered “high-grade” (HGG) [1]. Generally, LGG is less aggressive and confers a better overall prognosis. However, virtually all LGG tumors progress to HGG and eventually GBM which confers a particularly poor outcome. Despite resection with adjuvant chemoradiation having been shown to improve survival in patients with GBM, median survival is still approximately 14 months from the time of diagnosis [2]. Since care was standardized to adjuvant temozolomide and radiation 15 years ago, little progress has been made in improving the prognosis of glioma patients. However, several prognostic factors have been identified that have led to more accurate risk stratification. The relationship between extent of resection (EOR) and survival is one such prognostic factor that is becoming increasingly evident. Specifically, maximum resection of the contrast-enhancing portion of high-grade tumors confers longer overall survival [3][4][5][6][7]. Moreover, there is emerging evidence to suggest that even supra-total resection of regions beyond the contrast-enhancing margin may confer a survival benefit in GBM, further underscoring the importance of EOR in HGG [8]. The same relationship has been elucidated for LGG, although this is defined by mostly retrospective evidence [9][10].
A hallmark of low- and high-grade glioma alike is the propensity to infiltrate into surrounding tissue [11]. Unlike central nervous system (CNS) metastases which are generally well circumscribed and exert a mass effect on adjacent neurovascular structures, glioma invades and disrupts this anatomy, making en bloc gross total resection challenging. In HGG cases, attempts are made to resect the contrast-enhancing margin corresponding to contrast-enhancement on magnetic resonance imaging (MRI). Stereotactic navigation can be used to ensure that the resection margin extends to this contrast-enhancing border on preoperative imaging.
As visualizing the tumor margins for infiltrating regions of glioma in the operating room is difficult, techniques for intraoperative fluorescence have emerged to improve EOR in this patient population. 5-aminolevulinic acid (5-ALA) is the most prominent and well-studied of these and has been shown to increase EOR and progression-free survival in malignant glioma based on the results of a 2006 phase III trial [5]. The authors of this trial ultimately concluded that 5-ALA enabled more complete resection of the contrast-enhancing tumor. Of note, tumors with substantial non-contrast-enhancing regions, consistent with LGG, were excluded from analysis due to poor fluorescent response to 5-ALA administration. In fact, only 10–20% of LGG exhibited visible fluorescence with 5-ALA, making EOR in this patient population challenging [12][13]. Additionally, the use of 5-ALA has been associated with sporadic but not consistent fluorescence in some oncologic subtypes during resection, including metastatic lesions and lymphoma [14][15][16][17]. This indicates that in contrast to both LGG and other cranial tumors, the uniform and robust fluorescence found in high-grade gliomas represents a distinct and specific biological mechanism.
5-ALA is a non-proteinogenic amino acid formed through the condensation of succinyl-CoA and glycine that serves as a metabolic precursor for heme biosynthesis. In cells, 5-ALA is converted to protoporphyrin IX (PpIX), a photosensitizer and direct precursor to hemoglobin in the heme synthesis pathway. As a photosensitizer, PpIX is excited by light between λ = 405 (violet) and λ = 633 nm (red) [18]. The pathway for 5-ALA uptake and conversion to PpIX is outlined in Figure 1. Following initial discovery and synthesis of 5-ALA, it was tested for the diagnosis and treatment of skin, gastrointestinal, and bladder cancers, where it aided in disease recognition, excision, and photosensitizing treatment [19][20][21][22]. In 1998, Stummer and colleagues first described their results using oral 5-ALA to enhance intraoperative fluorescence of HGG which displayed high sensitivity, specificity, and accuracy for malignant cells [23]. Notably, their sentinel manuscript reported a lack of fluorescence in one patient with an LGG [23].
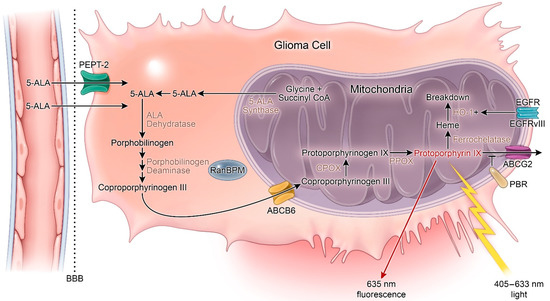
Figure 1. Heme synthesis pathway and mechanism of 5-aminolevulinic acid (5-ALA) fluorescence. 5-ALA: 5-aminolevulinic acid; ABCG2: ATP-binding cassette G2; ABCB6: ATP-binding cassette B6; BBB: Blood–brain barrier; CPOX: Coproporphyrinogen oxidase; HO-1: Heme-oxygenase 1; PBR: Peripheral benzodiazepine receptor; PPOX: Protoporphyrinogen oxidase. + = increased activity.
To build on this discovery, Stummer and colleagues went on to conduct a randomized phase III trial. They concluded that intraoperative use of 5-ALA in HGG was safe and markedly increased complete resection rates (65% in 5-ALA vs. 36% control). However, their study was underpowered to detect a difference in overall survival [5][24]. Based on these findings, the European Medicines Agency approved the use of 5-ALA in 2007. However, the United States Food and Drug Administration (FDA) approval took significantly longer due to their view of 5-ALA as a therapeutic rather than an adjunct tool to aid intraoperative visualization [25]. After the approval of an orphan drug application and the publication of other trials that highlighted the utility of 5-ALA in improving sensitivity and specificity of tumor margin discrimination during HGG resection, as well as progression-free survival at 6 months, the drug was approved for use in WHO grades III or IV by the FDA in 2017 [13][24][26][27][28].
5-ALA is the most studied of the photosensitizing agents in glioma and possesses many desirable properties for intraoperative tumor tissue discrimination. First, it is rapidly eliminated, reducing risk of skin sensitivity [29]. Indeed, early studies of 5-ALA metabolism demonstrated that approximately 80% of the substance was excreted in 24 h compared to 15N-labeled L-α-amino acids in rats [30]. Second, 5-ALA is readily bioavailable following treatment with oral formulations. In patients undergoing cranial surgery, patients are administered 5-ALA as a well-tolerated oral liquid 2–4 h before craniotomy. Third, 5-ALA accumulates in very few normal tissues (including mucosa of gastrointestinal tract, salivary glands, bile ducts, and seminal vesicles), thereby contributing to high signal to noise characteristics for tumor tissue visualization applications [21][31]. Fourth, 5-ALA is an endogenous human metabolite that is synthesized naturally from succinyl-CoA and glycine in the mitochondria; therefore, 5-ALA treatment is not associated with toxic side effects associated with administration of many xenobiotics. Taken together, these properties cooperate to render 5-ALA a valuable adjunct to neurosurgical HGG resection [32].
In normal cells, succinyl-CoA and glycine undergo condensation by 5-ALA synthase to form 5-ALA. 5-ALA is then converted through a series of enzymatic steps to PpIX and, ultimately, to heme. This process is regulated by free iron and heme. PpIX accumulation in the cell can result from (1) increased 5-ALA levels, (2) 5-ALA synthase hyperactivity, or (3) dysfunction of the ferrochelatase (FECH) enzyme that produces heme from PpIX [33]. Impaired tumoral FECH activity is one potential explanation for the specificity of PpIX accumulation in tumor tissues following 5-ALA treatment. Indeed, FECH has been shown to be decreased in colon carcinoma liver metastases compared to liver parenchyma in rat models following 5-ALA administration. Similar results were also reported in prostate cancer, bladder cancer, and colonic cells [34][35][36].
Porphobilinogen deaminase (PBGD) is another enzyme in the heme-synthesis pathway that promotes PpIX production. Increased levels of PpIX corresponding to increased activity of PBGD as well as ALA dehydratase and uroporphyrinogen decarboxylase have been reported in human breast carcinoma cells [37]. Similar results have been reported for squamous cell carcinoma and adenocarcinoma [38]. Additional evidence indicates that the feedback mechanism between PBGD and ALA dehydratase may play a central role in PpIX synthesis [39]. In 2002, Greenbaum et al. described a significant subcellular localization of PBGD in the nucleus that rapidly decreased following stimulation of C6 glioma cell differentiation [40][41]. The authors concluded that PBGD may have a unique nuclear function in glioma cells. One year later, these investigators identified the nuclear Ran-binding protein RanBPM as an interacting partner of PBGD [42]. They further concluded that nuclear localization of PBGD in glioma may be related to the process of malignant transformation in glioma. Although these results implicate altered activity of heme biosynthesis pathway enzymes as a general cause of 5-ALA-induced PpIX accumulation in malignant tissue, the mechanistic underpinning of this phenotype specifically relevant in HGG is an area of intense investigation.
Coproporphyrinogen oxidase (CPOX) is an enzyme of the heme synthesis pathway that generates protoporphyrinogen III (a direct precursor from PpIX) from coproporphyrinogen III via oxidative decarboxylation. Expression of this enzyme has been shown to directly correlate with PpIX fluorescence in human glioma cells [43].
In the setting of GBM, FECH expression has been shown to be reduced relative to normal brain tissue, leading to accumulation of PpIX. Importantly, FECH silencing via RNA interference is sufficient to promote this phenotype, thereby providing evidence of a functional connection between FECH repression and 5-ALA-induced PpIX accumulation in GBM [44]. Similar results linking PpIX accumulation and decreased FECH activity have been shown in medulloblastoma cell lines [45]. In addition to regulation at the level of gene expression, FECH can also be repressed by iron chelation. Reduced intracellular iron content blocks FECH activity and triggers PpIX accumulation in human adenocarcinoma and hamster lung fibroblast cell lines [46][47][48][49]. Differentiation therapy targets another enzyme, coproporphyrinogen oxidase, in the heme synthesis pathway, leading to increased PpIX concentration in prostate cancer cells [50]. Unfortunately, no differentiation therapies exist for malignant gliomas as these tumors are known to have a partial and abnormal potential for differentiation [52].
Further insight into the mechanism of 5-ALA fluorescence has been elucidated through studies of heme metabolism and its link to the tricarboxylic acid (TCA) cycle. Differential fluorescence upon 5-ALA treatment was observed in the IDH1R132H mutant compared to IDH1 wild-type (WT) WHO grade III gliomas. In IDH1R132H gliomas, the metabolite R-2-hydroxyglutarate (2-HG) accumulates, leading to cellular reprogramming and oncogenesis. The observation in gliomas was further explored in U87MG-IDH1R132H cells in comparison to U87MG-IDH1WT cells, where U87MG- IDH1R132H cell metabolism of 5-ALA to PpIX was delayed in contrast to U87MG- IDH1WT cells. As TCA cycle metabolites are involved in the generation of 5-ALA and thus PpIX, an exploration of the metabolic events that underlie the differences in 5-ALA mediated fluorescence was explored [51].
Upon 5-ALA treatment in U87MG-IDH1R132H cells, citrate and 2-HG concentrations were significantly increased, while α-ketoglutarate (α-KG) decreased relative to U87MG- IDH1WT cells. From these data, Kim et al. reviewed the interconnectedness of the TCA cycle and the heme biosynthesis pathway and hypothesized that nicotinamide adenine dinucleotide phosphate (NADPH)-dependent heme degradation is impaired, based on knowledge that the IDH1R132H mutation perturbs NADPH homeostasis [51][52]. Specifically, this mutation abrogates WT activity of the IDH1 enzyme, thereby blocking NADPH synthesis associated with the oxidative decarboxylation of isocitrate to α-KG. Furthermore, the IDH1R132H oncoprotein consumes NAPDH in the process of producing 2-HG [53]. Reduced levels of NADPH in IDH-mutated glioma cells has been proposed to contribute to PpIX accumulation [51]. Although this study highlighted metabolic alterations that lead to variance in 5-ALA-mediated fluorescence in IDH-mutated glioma cells, it is still not clear how IDH mutations influence glioma tissue fluorescence following 5-ALA treatment in the clinical setting. In particular, the simultaneous high prevalence of IDH mutations and low 5-ALA-induced tissue fluorescence associated with LGGs appear to be in conflict with the thesis that mutant IDH action stimulates PpIX content in glioma.
This conflict notwithstanding, there is additional evidence that NADPH production capacity may influence 5-ALA fluorescence due to crosstalk between the TCA cycle and heme synthesis pathway. Kim et al. conducted an RNA-sequencing study to compare GBM types classified by 5-ALA fluorescence [54]. This group identified glutaminase 2 as a regulator of 5-ALA fluorescence and confirmed this role in functional in vitro assays. Reduced expression of glutaminase 2 was associated with decreased production of NADPH and increased levels of PpIX and reactive oxygen species (ROS) in regions of GBM tumors with high fluorescence [54].
Lastly, lentiviral shRNA-mediated silencing of the heme biosynthesis enzymes in breast cancer cells was explored to better understand the pathway’s role in 5-ALA fluorescence. Porphobilinogen synthase (PBGS) and porphobilinogen deaminase are involved in the production of PpIX, whereas FECH degrades PpIX. Silencing of PBGS and PBGD led to decreased 5-ALA-mediated fluorescence and decreased sensitivity to photodynamic therapy (PDT), whereas silencing of FECH led to the opposite effects on fluorescence and PDT sensitivity [55].