1000/1000
Hot
Most Recent

Caesarean sections in obese patients are associated with an increased risk of surgical wound complications, including hematomas, seromas, abscesses, dehiscence, and surgical site infections. The aim of the present study is to perform a meta-analysis and systematic review of the current literature focusing on the strategies available to decrease wound complications in this population.
Obesity is a chronic disease that leads to the development of metabolic disorders and cardiovascular complications and currently poses a challenge for healthcare systems around the world [1]. According to the World Health Organisation (WHO), the problem of obesity is reaching epidemic proportions, in both developed as well as developing countries. In 2016, 15% of women over 18 years of age were either overweight or obese [2]. This problem also affects 1/3 of women of reproductive age [3], including 13% of pregnant women [4]. The rising prevalence of weight gain during pregnancy is associated with the occurrence of a greater number of complications during pregnancy, childbirth, or the postpartum period [5]. Research confirms that being overweight may also increase the rate of complications in both pregnant women and newborn babies [6]. Obese women develop arterial hypertension [7] and diabetes more often in pregnancy and undergo caesarean sections significantly more often [8], which means that they are diagnosed with postoperative surgical wound healing disorders more frequently [9].
Postoperative complications include: superficial infections, dehiscence, or the presence of a fluid reservoir (seroma and hematoma) at the wound site. The above symptoms concern 3 to 15% of women after a caesarean section [10][11] and often result in prolonged hospitalization, antibiotic therapy, thus leading to increased postpartum care costs. A superficial infection is part of a surgical site infection (SSI) which, according to the Centers for Disease Control and Prevention (CDC), is an infection that occurs within 30 days of the performed surgical procedure. The risk factors for the above complications include young age at childbirth, smoking, obesity, arterial hypertension, diabetes, chorioamnionitis, increased intrapartum blood loss, prolonged ruptured of membrane, emergency caesarean section and subsequent surgical delivery, use of suboptimal antibiotic prophylaxis, improper preparation of the surgical field, extended duration of the surgical procedure, and the employed caesarean section technique, including that of the incision and of the suturing of the skin [12][13][14][15].
Hypertension, like diabetes, is a disease that often coexists with obesity. Seven randomized trials investigate the effect of these diseases on wound healing [11][16][17][18][19][20][21], and take into account the premature rupture of the membranes, concomitant infections of the membranes or smoking. An American retrospective study found that 1 in 3 women who are morbidly obese will develop complications in the surgical wound, and smoking increases the risk of these complications by more than double (RR 2.7 [95% CI 1.08–6.54] p = 0.03) [16]. Another study confirmed that smoking and longitudinal skin incisions significantly increase the risk of infections and wound dehiscence after a caesarean section [21]. However, Temming and colleagues, in a study involving 1082 patients with a BMI of >30 did not confirm that obesity, smoking, diabetes, and chorioamnionitis had a significant impact on the risk of complications in the postoperative wound if the surgery was performed according to evidence-based medicine, that is, prophylactic antibiotics administered up to 60 min before the skin incision, the skin washed with an alcohol solution of chlorhexidine, the subcutaneous tissue sutured if its thickness is greater than 2 cm, and the skin sutured with an intradermal stitch. In this case, the only significant risk factor for wound disorders (27.5% vs. 16.1%, RR 1.71 [95% CI 1.12–247]) and the occurrence of SSIs (6.9% vs. 1.6%, RR 3.74 [95% CI 1.18–11.92]) is the fact of an emergency caesarean section [11]. Other authors showed no significant influence of diabetes, hypertension, the premature outflow of amniotic fluid or inflammation of the membranes on the increased incidence of postoperative wound complications in obese women [16][17][18][19][20].
The estimation of the optimal prophylactic dose of an antibiotic reaching a concentration higher than the minimum inhibitory dose (minimum inhibitory concentration)—both in the blood and in the adipose tissue—reducing the risk of postoperative wound infection, remains the subject of many studies [22][23][24][25][26][27][28][29][30][31][32]. In a 2011 study, Pevzner and colleagues questioned the effectiveness of a prophylactic dose of 2 g of cefazolin in women with varying degrees of obesity [22]. This was confirmed by Swank and colleagues, who showed that the minimal inhibitory concentration (MIC) was not reached in adipose tissue in relation to Gram-negative bacteria at the time of skin incision in 20% of obese women (BMI 30–40 kg/m2) and 44% of morbidly obese women after the use of cefazolin in a dose of 2 g. Increasing the dose to 3 g resulted in all the obese and 71% of the morbidly obese women reaching MIC ≥8 µg/mL [26]. In a randomized Australian cohort study involving 2231 women, increasing the dose of cefazolin to 3 g in women with a BMI of ≥30 kg/m2 resulted in a significant reduction in the occurrence of SSIs (OR 0.309, p < 0.001) [33]. In a randomized, double-blind study conducted among pregnant women in labor with a BMI of above 30, increasing the dose of cefazolin to 3 g did not significantly increase its concentration in adipose tissue and did not show significantly greater protection against the Staphylococcus species compared to the group receiving a dose of 2 g (61% vs. 72%, p = 0.35) [27]. In another retrospective US cohort study involving 335 obese women, increasing the prophylactic dose also did not reduce the incidence of SSIs [24]. In a randomized, double-blind study, Young et al. showed that although the concentration of the antibiotic in both blood serum and pregnant adipose tissue is dependent on the dose of the drug and the body weight, the use of both 2 g and 3 g of cefazolin is the optimal protection in the ratio of Gram-positive and Gram-negative bacteria [25]. Similar conclusions were provided by the study conducted by Groff and colleagues, which showed that the intravenous administration of 2 g of cefazolin fully protects against postoperative wound infections in both obese and normal-weight women. Moreover, this dose in both groups protects the newborn against an infection with the Streptococcus group B (GBS) and S. aureus [29]. Another American study investigating the penetration of an antibiotic into adipose tissue showed that the administration of 2 g of cefazolin 30–60 min before the skin incision reached concentrations above the minimum inhibitory concentration in both overweight and obese women if the procedure lasts less than 90 min (the probability of target attainment (PTA) in adipose tissue for 2 g of cefazoline was 92.4%, and 94.7% for 3 g of cefazoline). If the duration of the surgery exceeds 2 h, the PTA for 2 g of cefazoline was 86.8%, suggesting the need for another dose of antibiotic [30]. Eley et al. also confirmed that another prophylactic dose in the case of routine, uncomplicated patients is not needed [32]. However, Guper et al. showed that both the total body weight (TBW) and the BMI had no effect on the concentration of cefazolin in adipose tissue [30]. On the other hand, Kram and colleagues, in a study involving 84 patients with obesity of at least grade 1, found that its mean concentration in adipose tissue is still below the MIC of 8 mg/g (p < 0.03) regardless of the antibiotic dose used [31].
An incision of the skin and related complications within the postoperative wound were the subject of six retrospective randomized studies in which the incidence of complications following a transverse or vertical skin incision was analyzed. Most authors take into account the risk of wound infection and its dehiscence, as well as the presence of fluid collections (seroma and hematoma) resulting in rehospitalization. In a US cohort study of morbidly obese pregnant women, the risk of postoperative wound complications following a vertical abdominal incision more than doubled (OR 2.2 (1.18–4.27)) [16]. Another study involving 242 women with a BMI of ≥30 kg/m2 indicated that obesity significantly increases the risk of complications within a postoperative wound, although the method of skin incision had no effect on the incidence of this complication [17]. Smid et al., in an analysis of 2411 pregnant women, showed that those with morbid obesity (BMI > 45 kg/m2) are at increased risk of endometritis (AOR 1.26; 95% CI 1.07–1.49) and wound infections (AOR 3.77; 95% CI 2.60–5.46) compared to women with normal body weight. Moreover, he showed that infections accompany vertical incisions of the skin significantly more often (p = 0.02) than transverse incisions [34]. Thornburg et al. assessed that vertical incisions increase the risk of wound complications by more than seven-fold. There is an increased risk of infection (OR 5.16; 95% CI 2.3–11.8) and wound dehiscence (OR 10.7; 95% CI 4.0–29.2) in obese women, regardless of the degree of their obesity [18]. In the study from US, the authors showed a significantly lower incidence of complications within the wound (including infection, seroma, hematoma, and fascial dehiscence) in the case of vertical incisions of the skin (OR 0.32; 95% CI 0.17–0.62) [35].
The impact of the type of skin incision on the risk of wound complication in obese women was assessed based on six studies with a total number of 928 women with vertical skin incision and 1522 obese women with transverse skin incision. Pooling the data together, we observed that wound complications were present in 21% of obese women with vertical incision but only in 12% of obese women with transverse incision. Studies were mildly moderately heterogeneous (I2 46% with p = 0.10) thus the FEM method was used to calculate the pooled OR. Wound complications were found to be significantly more frequent in women with vertical skin incision than in women with transverse skin incision (21% vs. 12%). The value of pooled OR equals to 2.48 (95%CI 1.85–3.32, p < 0.01) indicates that the odd of wound complication in obese women after a caesarean section is almost 2.5-fold higher in the case of vertical skin incisions (Figure 2). No publication bias was found for this analysis [Egger’s test (p = 0.633); Begg’s test (p = 0.348)] and sensitivity analysis demonstrated that the results were stable and reliable.
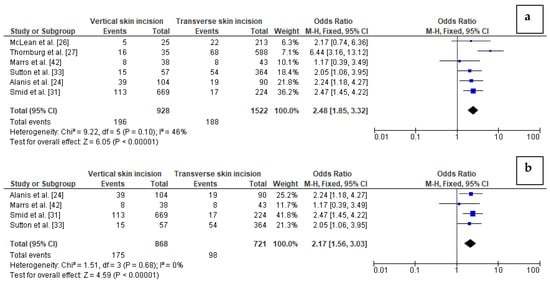
Figure 2. Forest plots for: (a) wound complications in the group of post-caesarean obese women with vertical skin incision and in women with transverse skin incision; (b) wound complications in the group of post-caesarean women with BMI ≥40 kg/m2 and vertical skin incision compared to women with transverse skin incision. M.-H.—Mantel-Haenszel; CI—confidence interval; I2—heterogeneity; df—degrees of freedom.
In addition, we performed subgroup analysis based on four studies considering women with BMI ≥ 40 kg/m2, including 868 women with vertical skin incision and 721 women with transverse skin incision in total. There was no heterogeneity between the studies (I2 0%). The analysis revealed that wound complications were again significantly more frequent in women with extreme obesity and vertical skin incision than in extremely obese women with transverse skin incision (20% vs. 14%, respectively). The results showed that the odd of wound complication in women with BMI ≥40 kg/m2 after caesarean section is almost 2.2-fold higher in the case of vertical skin incision than transverse skin incision (OR = 2.17 (95%CI 1.56–3.03, p < 0.01) (Figure 2). However, in this analysis publication bias was observed [Egger’s test (p = 0.008); Begg’s test (p = 0.041)]. In turn, sensitivity analysis showed that the results of OR calculation were stable.
Four randomized trials compared the effectiveness of stitching skin with staplers, single sutures, and intradermal sutures. An Egyptian study conducted among 130 women diagnosed with obesity whose skin was closed with an intradermal suture showed a significantly higher risk of SSIs and postoperative wound pain but a significantly better cosmetic effect was achieved. The use of staplers in suturing the skin shortens the duration of the surgical procedure; although it is associated with an increased risk of wound dehiscence in obese women. RR 5.2 (95% CI 1.8–14.7) [36]. Similar results were obtained in a randomized cohort study involving 1147 women, in which sewing the skin with the staplers more than doubled the risk of postoperative wound dehiscence (RR 2.20; 95% CI 1.6–3.1) and contributed to its infection (wound infection or cellulitis) (RR 2.46; 95% CI 1.4–4.4) compared to the group of women who received intradermal sutures [19]. However, a 2018 study of women with grade III obesity did not confirm the above results [20].
The prophylactic drainage of subcutaneous tissue aims to reduce the risk of formation of fluid reservoirs within a wound, which could disrupt its continuity or lead to its infection. The studies conducted by Al-Inany et al. [37] and Ramsey et al. [38] did not confirm the effectiveness of the prophylactic drainage of subcutaneous tissue. Alanis, in a retrospective cohort study, concluded that prophylactic drainage of subcutaneous tissue should be abandoned in women with massive obesity [16]. An American randomized cohort study showed that the drainage procedure significantly increases the risk of wound complications (OR 2.86; 95% CI 1.02–7.98), contributing to both its dehiscence and infection [18]. A study by Bindal and Munda based on obese women from India demonstrated that patients after a caesarean section with a drain had reduced rates of wound seroma, postoperative pain, and shorter hospital stays [39]. However, the authors did not observe any significant benefits of the drainage with regard to postoperative fever, superficial SSI, as well as wound breakdown [39]. A retrospective cohort study by Dias and colleagues [40] performed on severely obese women with a BMI of >40 kg/m2 showed no correlation between the use of a drain and SSI. A Japanese study analyzing two groups of women with a mean BMI of approx. 33 kg/m2, the first of which had staples and the second had subcuticular sutures and drains [41] found that the frequency of wound complications was significantly lower in women with a drain as compared to those with staples. The authors used four-channel Blake drains, which are made from soft fluted silicone with a wide surface area for drainage. This type of drain was demonstrated to be less painful in comparison to non-Blake drains [42]. On the other hand, Aziz Khalifa and colleagues reported a significant difference between obese women with a seroma drain after a caesarean section and women with no seroma drain (9.6% vs. 26.7%, respectively) and postoperative pain requiring analgesics [43]. Most of the available data regarding the usage of drains in post-caesarean women were obtained from studies performed on a low number of patients, which may have influenced the results. Thus, performing a meta-analysis may overcome the effect of a small analyzed population. To the best of our knowledge, no meta-analysis on the correlation between drainage use and wound complications in obese women after caesarean sections have been performed. The meta-analyses of Gates published in 2005 and 2013 [44] included studies analyzing women with both a normal BMI and overweight or obese women.
In the present study, we performed a meta-analysis concerning the comparisons of surgical complications after caesarean sections in obese women in terms of wound complications (including data on wound separation), infections (including data on SSI), as well as fevers.
In general, nine studies with a total number of 674 obese post caesarean women with a drain and 1718 obese women with no drain were included in the meta-analysis [45][37][38][16][41][18][43][39][40]. The impact of drainage on the risk of wound complications in obese women was conducted based on eight studies with a total number of 658 women with a drain and 1,283 obese women with no drain. Studies were highly heterogeneous (I2 84%). The percentage of wound complications was slightly higher in obese women with a drain (21%) compared to obese women without a drain (19%). Since significant heterogeneity between studies was calculated, the REM method was used to calculate the pooled OR. The difference was not significant (OR 1.32; 95% CI 0.64–2.70, p = 0.45), which indicates that the use of drains does not increase the risk of wound complications in obese women after a caesarean section (Figure 3).
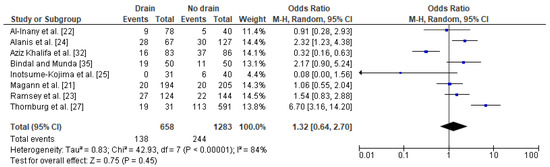
Figure 3. Forest plot for wound complications in the group of post-caesarean obese women with a drain and in the group of obese women without a drain. M.-H.—Mantel-Haenszel; CI—confidence interval; I2—heterogeneity; df—degrees of freedom.
The results were stable after subsequent omitting each of the studies that was included. No publication bias was found for this analysis [Egger’s test (p = 0.577); Begg’s test (p = 0.458)].
The relation between drainage and infections (including SSI) after a caesarean section in obese women was based on five studies with a total number of 258 women with a drain and 1202 obese women with no drain. The percentage of wound complications was slightly lower in obese women with a drain (8%) as compared to obese women without a drain (13%). The heterogeneity between studies were at low level (I2 27%) with no significance, therefore FEM method was used to calculate the pooled OR. The difference was not significant (OR 0.93; 95% CI 0.53–1.63, p = 0.80), which indicates no impact of drain usage on infections after a caesarean section in obese women (Figure 4). The results were stable during the sensitivity analysis; thus, the analysis is reliable. Again, no publication bias was observed [Egger’s test (p = 0.056); Begg’s test (p = 0.142)].
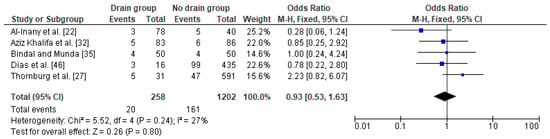
Figure 4. Forest plot for infections in the group of post-caesarean obese women with a drain and in the group of obese women without a drain. M.-H.—Mantel-Haenszel; CI—confidence interval; I2—heterogeneity; df—degrees of freedom.
In the case of the analysis of the impact of drainage on a fever after a caesarean section in obese women, three studies were included with a total number of 211 women with a drain and 176 obese women with no drain. The proportion of fevers was slightly lower in obese women with a drain (16%) as compared to obese women without a drain (20%). In this analysis, no heterogeneity between the studies was demonstrated (I2 0%) and the FEM method was once again used to calculate the pooled OR. The difference was close to the border of significance (OR 0.62; 95% CI 0.36–1.07, p = 0.09) indicating that drain usage after a caesarean section may have some beneficial effect in the presence of a fever in obese women (Figure 5). The sensitivity analysis revealed that the results were stable and reliable and no publication bias was revealed [Egger’s test (p = 0.342); Begg’s test (p = 0.601)].
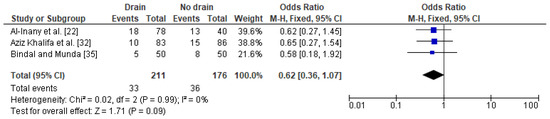
Figure 5. Forest plot for fever in the group of post-caesarean obese women with a drain and in the group of obese women without a drain. M.-H.—Mantel-Haenszel; CI—confidence interval; I2—heterogeneity; df—degrees of freedom.
Seven randomized trials concerning the effectiveness of vacuum dressings in the prevention of SSIs were carried out. Most of the studies indicated the ineffectiveness of the prophylactic use of the above therapy. The study conducted in women with a BMI of ≥30 kg/m2 did not show the negative pressure wound therapy (NPWT) dressing to significantly reduce the incidence of complications in the postoperative wound compared to standard wound care (4.9 vs. 6.9%; p = 0.71) [46]. Similar results were provided by the Hussama study that included 441 women with morbid obesity (BMI >40 kg/m2), where a closed vacuum dressing was used as compared to standard wound care, and also did not significantly reduce the incidence of complications in the skin (RR 0.9 [95% CI 0.5–1.4]) [47]. However, a Danish study from 2019 that encompassed 876 women with class I obesity or greater showed that SSIs were found in 4.6% in the NPWT dressing group, while in the control group there were 9.2% SSIs (RR 0.50; 95% CI 0.30–0.84); the number needed to treat 22; p = 0.007). The authors found that vacuum dressings used prophylactically in obese women significantly reduced the incidence of SSIs [47].
Meta-analysis of the data regarding the frequency of SSI in obese women with NPWT in comparison to women with standard therapy was based on six studies with 1835 cases with NPWT therapy and 1842 patients with standard therapy. One of the studies was excluded because of incomplete data (absence of patient with standard therapy) [48]. This revealed that the prevalence of SSI was lower among obese women with NPWT than in women receiving standard wound therapy (8% vs. 10.5%, respectively). A significant reduction in the SSI odds ratio was observed in obese women with negative pressure wound therapy compared to obese women with standard therapy (OR = 0.76 95% CI 0.60–0.95, p = 0.02) (Figure 6). Very low level of heterogeneity between the studies was observed (I2 = 7%).
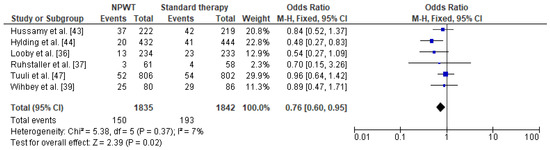
Figure 6. Forest plot for surgical site infection in the group of post-caesarean obese women with NPWT and in obese women with standard therapy. NPWT—negative pressure wound therapy; M.-H.—Mantel-Haenszel; CI—confidence interval; I2—heterogeneity; df—degrees of freedom.
The results were stable after omitting one study at a time, i.e., the study by Hussamy et al. [47] first, then a study by Ruhstaller et al. [46], then a study by Wihbey et al. [49], and then a study by Tuuli et al. [50]. When the study by Hylding et al. [51] was omitted, the results changed and were not significant (OR = 0.84 95% CI 0.65–1.08, p = 0.17). Similarly, omitting the data by Looby et al. [52] gave insignificant results (OR = 0.79 95% CI 0.62–1.01, p = 0.06). Therefore the result of the meta-analysis should be treated with caution. The results of Egger’s and Begg’s tests showed no publication bias [Egger’s test (p = 0.456); Begg’s test (p = 0.348)].
The use of a prophylactic dose of antibiotic in women undergoing a caesarean section reduces the risk of postoperative wound infections (RR 0.40; 95% CI 0.35–0.46, 82) and inflammation of the endometrial mucosa (endometritis) (RR 0.38; 95% CI 0.34–0.42, 83), as well as other complications resulting from infections (RR 0.31; 95% CI 0.20–0.49) [53]. The time of administration of a prophylactic dose of antibiotics also affects the frequency of infection. The studies show that the administration of an antibiotic before a surgical procedure reduces the percentage of endometritis by 41% compared to the intraoperative administration of an antibiotic (RR 0.59; 95% CI [95% CI] 0.37–0.94; I2 0%), although such treatment does not reduce the incidence of postoperative wound infections (RR 0.71; 95% CI 0.44–1.14; I2 0%) [54]. In clinical practice, the 1st-generation cephalosporin, Cefazolin, at a dose of 2 g, which has a spectrum of activity including Gram-positive bacteria and Escherichia coli, is used in the prophylaxis of perioperative caesarean sections. As evidenced by studies conducted among obese women who were not pregnant, cefazolin shows different pharmacokinetics in obese people, which means that its concentration is lower in adipose tissue. This phenomenon suggests that the prophylactic dose needs to be increased in such a group of obese patients [55]. The studies that we analyzed did not confirm the effectiveness of a higher dose of antibiotic in perioperative prophylaxis.
The suturing of subcutaneous tissue aims to reduce the risk of formation of fluid reservoirs. A meta-analysis of six studies showed that suturing reduces the risk of wound complications by 34% [56] in subcutaneous tissue that is thicker than 2 cm. A randomized study of 1082 women undergoing a caesarean section treated with antibiotic prophylaxis for more than 60 min from the skin incision, with the skin washed with an alcoholic chlorhexidine solution, and sutured subcutaneous tissue if its thickness was greater than 2 cm, as well as skin closed with sutures instead of staplers, showed a significant reduction in the risk of complications in the postoperative wound, regardless of the method of incision of the skin, the coexistence of obesity and diabetes, chorioamnionitis or the operator’s experience [11]. However, the studies analyzed by us and the meta-analysis on the method of incision of the skin demonstrated that a Pfannenstiel caesarean section reduces the risk of postoperative wound infections [57].
The prophylactic use of vacuum dressings in obese women is aimed at reducing the incidence of SSIs. As shown by a meta-analysis of seven studies, the prophylactic use of NPWT reduces the risk of SSIs (pooled RR 0.45; 95% CI 0.31, 0.66). Complex wound complications were significantly reduced in patients receiving prophylactic negative pressure wound therapy compared to standard dressings (9 studies: pooled RR 0.68; 95% CI 0.49, 0.94) [58]. Another meta-analysis conducted on 10 studies from 1966 to 2017 did not confirm that NPWT decreased wound complication (RR 0.97, 95 % CI 0.63–1.49) [59]. Moreover the most recent American study based on 1624 people also did not significantly confirm NPWT in reducing SSIs. [50] The analysis carried out by us, supplemented with the current 6 researches, showed, that NPWT reduces the risk of SSIs, nevertheless, the results should be treated with caution because the sensitivity analysis was not stable and no significance was observed after omitting the studies by Hylding et al. [51] and Looby et al. [52] no significance was observed.
The prophylactic use of subcutaneous drains to reduce SSIs remains controversial. The meta-analysis concerning the use of subcutaneous tissue drainage after surgical procedures did not show a reduction in incidence of complications in the postoperative wounds; hence, it is unjustified in everyday surgical practice [60], as well as in caesarean sections and other surgical procedures performed in obese patients [61], which was confirmed in our meta-analysis.
The strength of our study is the meta-analysis of the research on the prophylactic use of a subcutaneous drain during a caesarean section procedure in obese women. It should be noted that no such analysis has been carried out to date. In addition, current research on the NPWT confirms that SSIs are significantly infrequent in obese women with NPWT, however, further analysis should provide more studies to confirm stable and reliable results.
The relatively small number of studies and significant variability in outcome reporting are important limitations of our study. All analyzed studies by us were in English language, which also might be a limitation of the study. Moreover, a considerable amount of research concerning the issue of caesarean sections includes retrospective studies that do not comprise clinical indications for a surgical procedure and do not refer to data regarding the duration of amniotic fluid leakage, the presence of uterine contractions, and a history of diabetes. Furthermore, the authors of some studies did not differentiate between caesarean sections in schedule and emergency ones [10][17][20][62]. The heterogeneity of the studies included in the review also constitutes a limitation of the study.