1000/1000
Hot
Most Recent

The interplay between drugs and microbiota is critical for successful treatment. An accumulating amount of evidence has identified the significant impact of intestinal microbiota composition on cancer treatment response, particularly immunotherapy. The possible molecular pathways of the interaction between immune checkpoint inhibitors (ICIs) and the microbiome can be used to reverse immunotherapy tolerance in cancer by using various kinds of interventions on the intestinal bacteria. This paper aimed to review the data available on how the antibiotic-related changes in human microbiota during colorectal cancer (CRC) treatment can affect and determine ICI treatment outcomes. We also covered the data that support the potential intimate mechanisms of both local and systemic immune responses induced by changes in the intestinal microbiota. However, further better-powered studies are needed to thoroughly assess the clinical significance of antibiotic-induced alteration of the gut microbiota and its impact on CRC treatment by direct observations of patients receiving antibiotic treatment.
The interplay between drugs and microbiota is critical for successful treatment. Therefore, pharmacomicrobiomics was proposed as a modern approach for assessing the interaction between medicines and microbiota composition. A growing amount of evidence suggests that intestinal microbiota composition significantly impacts cancer treatment response, particularly immunotherapy. It has been shown that specific intestinal bacteria may promote or suppress immune checkpoint inhibitor (ICI) effectiveness. The possible molecular pathways of such an interaction can be used to reverse immunotherapy tolerance in cancer by using various kinds of interventions on intestinal bacteria [1].
However, the intestinal microbiome can alter bioavailability, activity, and toxicity by transforming medication [2]. This widely-accepted concept lays stress on the importance of the balance between beneficial and harmful bacteria rather than the presence or absence of individual bacterial species. Numerous factors have been identified as altering temporarily or permanently the structure of intestinal microbiota, the key ones being diet, antibiotics, other pharmaceuticals, immune system factors, and cancer [3].
Changes in the typical intestinal microbiota composition have been linked—but not confined—to inflammation, a well-known cancer trait. The microbiome–immune system–cancer axis needs further evaluation, especially regarding microbiota’s influence on different cancer therapies, including immunotherapy. Targeted immune-mediated treatments have been a crucial point in recent developments regarding solid tumor treatment. ICIs have been one of the most significant groups of treatments to impact the whole field of oncology. ICIs have demonstrated clinical benefits in various cancers, including colorectal cancer (CRC) with microsatellite instability (dMMR/MSI-H) in the metastatic setting [4][5]. Therefore, the increasing role of ICIs in treating cancer has led to further analyses of the factors predicting their response—mostly, which factors can lead to failure of ICI treatment.
Direct and indirect alterations of the microbiome composition and metabolic capacity as a consequence of antibiotic usage have been examined and documented thoroughly [6]. However, changes in the gut microbiota caused by antibiotics may also alter the number and functions of immune cells in the intestines, leading to systemic inflammatory responses in the body [7]. In line with this, it is not surprising that the effectiveness of immunotherapy may vary in response to alterations in microbiota composition [8][9].
The intensive crosstalk between the host gut microbiota and the intestinal immune system is essential for the maturation of immune cells. The human microbiome is also involved in education and enabling the immune system to recognize potentially hazardous bacteria, thus avoiding invasion and infection.
The effect of the gut microbiome on the response to immune checkpoint blockade of different cancer types has been shown [10]. In regard to CRC, it is considered that the aggressiveness and treatment response are determined by various immune cells inside the tumor and in the tumor microenvironment. It has been established that lymphoid and myeloid cells, fibroblasts, and endothelial cells are involved. The critical participation of cytotoxic CD8+ T cells, dendritic cells, tumor-associated macrophages, and cancer-associated fibroblasts play a central role as modulators and even drivers of tumor heterogeneity [11]. The immune cell populations within the tumor and the tumor environment are not the only players that encourage tumor development. It has been documented that gut microbiome dysbiosis with loss of protective bacterial populations and the enrichment of microbial communities can encourage cancer. Therefore, it is essential to understand the dynamic changes in CRC patients’ gut microbiome to clarify the whole process of CRC carcinogenesis.
Recent studies have shown that patients with CRC have an altered gut microbiome compared to healthy controls. For example, an overabundance of several intestinal bacterial organisms, including Fusobacterium nucleatum, enterotoxigenic Bacteroides fragilis (ETBF), and Peptostreptococcus anaerobius, have been reported to promote tumor proliferation in CRC carcinogenesis [12]. Furthermore, the correlation between the bacteria mentioned above and inflammation and the tumor shield for immune attack has been suggested.
The critical effect of commensal microbes on cancer patients’ prognosis has also been reported in recent studies. Mima et al. revealed the association between the abundance of Fusobacterium nucleatum DNA in CRC tissue and a shorter survival in a large patient cohort study. Moreover, the amount of F. nucleatum DNA may potentially serve as a prognostic biomarker in clinical outcomes [13]. Moreover, Yu et al. revealed the role of F. nucleatum in fostering chemoresistance in patients with CRC by inducing autophagy, which fails treatment or provokes disease recurrence [14]. Recently, we gathered data on F. nucleatum and its connection with microbiota dysbiosis, the progression of CRC, the transformation of conventional adenoma to CRC, and the serrated carcinoma pathway [15].
One of the prominent causes that increases the risk of accelerated proliferation and deleterious mutations is the degradation of mucin by pathogens, resulting in biofilm formation on the epithelial surface and adverse immune responses [16]. Thus, one would speculate that biofilm invasion deep into colonic crypts would accelerate carcinogenesis. In patients with biofilms, the risk of developing CRC is higher compared to those without biofilms. These bacterial biofilms are also observed in normal colon mucosa, but have been associated with decreased colonic epithelial cell E-cadherin expression and increased activation of IL-6 and STAT3 in epithelial cells, as well as increased proliferation of crypt epithelial cells [16]. We also recently established IL-6 as a crucial cytokine of equal importance for both inflammation and tumor development, suggesting that IL-6 is a significant tumor promoter during the early stages of CRC [17].
Although the exact mechanisms remain to be discovered, a growing number of preclinical animal model studies and clinical trials of immunotherapies suggest that the host microbiome is a critical determinant for the variable host responses to different therapy modalities [18]. Yu et al. demonstrated that the same type of pro-inflammatory cells induced due to dysbiosis leads to increased T cell exhaustion in the tumor microenvironment [19]. Gut microbiota dysbiosis also increases the intestinal barrier’s permeability, favoring bacterial translocation, macrophages activation, and the consequent establishment of chronic pro-tumorigenic inflammation.
Studies have already identified specific commensal flora members that exert significant microbiome-dependent control on anti-tumor immunity, including immune system priming and the response to ICIs. However, it is difficult to identify the single most significant member of the flora for tumor favoring. Still, several species contribute to cancer through different mechanisms, and some species have detrimental effects on anti-tumor treatment. It has been demonstrated that normal gut microbiota might enhance the anti-tumor activity of ICIs by promoting the secretion of IL-12 by local dendritic cells and by changing the local repertoires of Th1 cells to express the intestinal chemokine receptors CCR9 and CXCR3. It has been reported that the anti-tumor potential of CD8+ cells is also affected by the intestinal microbiota, where anti-tumor mechanisms depend on increased IFN-γ production and CD8+ count within the tumor [9][20]. For example, Bifidobacterium has been shown to improve anti-tumor immunity in vivo, both alone and in combination with anti-PD-L1 immunotherapy through modulation of dendritic cell function and the subsequently improved effector function of tumor-specific CD8+ T cells [21].
Furthermore, lysates of Lactobacillus acidophilus also have enhanced anti-tumor efficacy of anti-CTLA-4 (cytotoxic T lymphocyte-associated protein 4) blocking antibody in mice CRC models, associated with increased CD8+ T cells, decreased numbers of T regulatory cells (Tregs), and decreased M2 macrophages in the tumor microenvironment [22]. Interestingly, bacterial genotoxins from Bacteroides fragilis, Campylobacter jejuni, and Fusobacterium nucleatum could promote CRC development in patients via activation of CD4+ Th17 cell responses, mTOR signaling, and the NF-kB pathway, respectively [23][24]. Additionally, bacteria such as ETBF and Fusobacterium nucleatum can impair the effectiveness of traditional chemotherapy and ICIs. The variations in treatment results between right-sided and left-sided colon cancer can be explained by biofilms in the right colon [16], as discussed above.
The human intestinal flora has been subject to vigorous studies in recent years, mainly due to the advances in genetic techniques allowing for sequencing studies of unculturable bacteria. Metagenomic analyses of gut microbiome changes induced by antibiotics still involve mostly small (<100) cohorts of patients/volunteers, allowing much room for intragroup variability. These limitations were the primary and intrinsic weaknesses of the above analyses. Moreover, metagenomic analyses from animal models of the human intestinal microbiota are not a suitable replacement; for some more recent classes of antibiotics, such as carbapenems and polymixins, human metagenomic data are scarce, if any. Further work on larger populations will be needed to affirm the trends.
However, advanced technologies have allowed thorough examination of the gut microbiota’s genera level, demonstrating the domination of the taxa Bacteroides and Firmicutes, compared to relatively lower shares of Proteobacteria, Actinobacteria, and Fusobacteria [25].
Thus, antibiotics exert various neglected side effects on gut microbiota, affecting immune cell development, function, and regulation. When antibiotics diminish the beneficial gut microbiota, changes in the numbers and function of naïve cells, Th1/Th2 cells, Th17, and T regulatory cells occur [26]. Some of these alterations exert systemic effects in the organism, such as increased susceptibility to infections and sepsis. The systemic immune dysfunction found in antibiotic-treated patients is associated with impaired defense against pathogens, dysregulated toll-like receptor (TLR) signaling, reduced expression of antimicrobial peptides, low IgA production in the mucosa, decreased expression of IFN-γ (causing impaired clearance of viruses), etc. [27]. Except for the main drugs altering the microbiome, i.e., antibiotics, several other common non-antibiotic medicines have been related to disrupted gut microbiota composition and function.
In line with this, the microbiome changes during antibiotic therapy that affect the immune system may influence the immunotherapy efficacy, including ICIs for CRC. The issue is complicated because one has to analyze how different antibiotics impact the intestinal flora and how they interact with the immune mechanisms, especially in cancer and cancer treatment.
Claesson et al. focused on general antibiotic usage (regardless of the class of antibiotics). They discovered a trend of shifting the balance toward Bacteroides at the expense of Firmicutes, Actinobacteria, and Proteobacteria [28]. The same study analyzed the use of a broad wide-spectrum macrolide antibiotic (clarithromycin) explicitly in a short time frame of three months. They found that this specific antibiotic shifted the balance toward Firmicutes at a lower dose of 250 mg and away from major phyla at a higher dose of 500 mg. Furthermore, the study illustrated that the balance between favorable and unfavorable bacteria in terms of anti-tumor immune modulation is variably disturbed by different antibiotic classes, and even by their doses [28].
Another small study assessed the effect of a second-generation cephalosporin (cefprozil) on healthy volunteers’ intestinal flora, which is also a widely used antibiotic in outpatient settings. The findings of the study demonstrated high individual variability of the response to the treatment. The trends showed a reduction of Bacteroides representatives, and an increase of E. coacae and Lachnoclostridium bolteae [29]. The study also observed incomplete recovery to the initial state in several of the exposed subjects. Thus, lower microbiome diversity and the prevalence of Bacteroides enterotype may remain long after the antibiotic treatment.
A deeper look into the available data for alterations of the human microbiota under antibiotic treatment shows that the composition changes mostly follow the pattern of intrinsic resistance of the respective families to the individual antibiotics. Although there is evidence of the beneficial and detrimental members of the intestinal flora in view of the ICI therapy, data regarding species that are not pathogenic are limited in terms of antibiotic susceptibilities and are particularly problematic for unculturable or hardly culturable species such as Akkermansia muciniphila.
The relationship between medicines and the microbiome is further extended by allowing some medications for CRC to be metabolized by microbiota into toxic metabolites or inactive molecules. For example, the drug availability and efficacy of monoclonal antibodies against PD-1 (programmed cell death protein 1) and its ligands PD-L1 and CTLA-4 may depend on gut microbiota composition [10][30].
A healthy microbiome has anti-tumor activities that can also impair the therapeutic success of the treatment, including cancer therapy with ICIs. Cancer immunotherapy can be enhanced or suppressed by the gut microbiome’s overall effects on the host immune system [31]. Furthermore, Routy et al. demonstrated that the use of antibiotics before, during, and after PD-L1 or PD-1 inhibition is linked to poorer prognosis and decreased progression-free survival [32]. Other anti-cancer drugs, such as daunorubicin and 5-fluorouracil, are associated with antimicrobial activity themselves [33].
Thus, standard chemotherapy success is often based on intact immune responses. These data support the hypothesis that intestinal microbiota can also modulate the therapy types. Therefore, cancer treatment success might depend on immune and microbiome interactions.
A schematic picture of ICI interactions with microbiota and immune cells is presented in Figure 1.
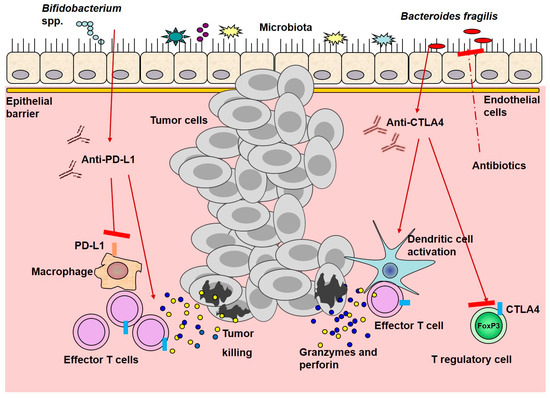
Figure 1. Immune checkpoint inhibitor interactions with microbiota and immune cells. Anti-PD-L1 treatment cooperates with resident Bifidobacterium spp., leading to activation of dendritic cells, promoting the activation, expansion, and function of T effector cells. Anti-CTLA4 promotes the enrichment of resident Bacteroides spp. And enhances dendritic and effector T cell activation, while suppresses T regulatory cells function. All of these mechanisms improve anti-tumor efficacy, in contrast to antibiotics that might decrease it. Red arrows represent stimulation, whereas red arrows with red rectangle—inhibition.