1000/1000
Hot
Most Recent

Fabric phase sorptive extraction (FPSE) is an evolutionary sample preparation technique which was introduced in 2014, delivering all green analytical chemistry (GAC) requirements by implementing a natural or synthetic permeable and flexible fabric substrate to host a chemically coated sol–gel organic–inorganic hybrid sorbent in the form of an ultra-thin coating. This construction results in a versatile, fast, and sensitive micro-extraction device. The user-friendly FPSE membrane allows direct extraction of analytes with no sample modification, thus eliminating/minimizing the sample pre-treatment steps, which are not only time consuming, but are also considered the primary source of major analyte loss. Sol–gel sorbent-coated FPSE membranes possess high chemical, solvent, and thermal stability due to the strong covalent bonding between the fabric substrate and the sol–gel sorbent coating. Subsequent to the extraction on FPSE membrane, a wide range of organic solvents can be used in a small volume to exhaustively back-extract the analytes after FPSE process, leading to a high preconcentration factor. In most cases, no solvent evaporation and sample reconstitution are necessary.
When an analytical or bioanalytical chemist is presented with a sample for analysis, regardless of the nature of the sample, a number of important decisions must be made such as which chromatographic/electrophoretic instrument will be used and what the sample preparation strategy will be, among others. Unless the analyst is imposed with some regulatory restrictions, the analyst may independently decide as to whether a solvent based extraction technique (e.g., liquid–liquid extraction, liquid phase microextraction) or a sorbent based extraction technique (e.g., solid phase extraction, solid phase microextraction, stir bar sorptive extraction) [1][2][3] will be deployed. If the goals of the sample preparation are to achieve highly selective extraction of the target analyte(s) as well as to minimize the matrix interference, the obvious choice would be sorbent-based extraction techniques. Subsequently, another major decision point would be whether the sample preparation technique is an exhaustive one as used in solid phase extraction (SPE), or an equilibrium driven one as used in solid phase microextraction (SPME). Both the techniques have some advantages and shortcomings. In addition to the differences in the extraction mechanism, they use almost exclusively two different sets of sorbents (with a few exception). SPME and its different modifications can be deployed in the field, whereas SPE is not generally field deployable. What if an analyst wants to exploit all the advantageous features of both the techniques while minimizing the inherent shortcomings? Keeping this dilemma in mind, Kabir and Furton [4] developed fabric phase sorptive extraction in 2014 as a new generation sample preparation technique that innovatively combines both SPE and SPME in a single sample preparation technology platform. Fabric phase sorptive extraction (FPSE) simultaneously exerts exhaustive extraction mechanism as well as equilibrium driven extraction during the sample preparation process and consequently accomplishes exhaustive or near exhaustive extraction even when the extraction is carried out under equilibrium extraction conditions (e.g., direct immersion extraction). As such, FPSE is neither a new format of SPME nor a new format of SPE, but a true combination of both the techniques.
FPSE has not only combined the extraction mechanisms of SPE and SPME, it has also successfully made available all the sorbents which are exclusively used in either SPE or in SPME. For example, poly(dimethylsiloxane), PDMS, is a popular sorbent coating used in SPME. On the other hand, the C18 phase is predominantly used in SPE. Now, an analyst may use both the sorbents in FPSE.
FPSE is the first sample preparation technology that exploits the surface chemistry of the substrate. In fact, the selectivity and the extraction efficiency of the FPSE membrane originate from the organic polymer, one or more organically modified inorganic precursor and the surface chemistry of the fabric substrate. As such, the selectivity and extraction efficiency of sol–gel PDMS coating on cellulose fabric is substantially different from that of sol–gel PDMS coating on polyester or fiberglass fabric.
FPSE also enjoys the enormous advantages of sol–gel synthesis process that chemically binds the organic polymer/ligand to the substrate using an inorganic/organically modified linker. The chemical bonding between the substrate and the polymer assures very high thermal, solvent, and chemical stability of the FPSE membrane. As a result, the FPSE membranes can be exposed to pH 1–13, as well as to any organic solvent without compromising structural and chemical integrity of the extracting polymer. Sol–gel based chemical coating process provides unprecedented batch-to-batch reproducibility. It is worth mentioning that classical extraction and microextraction techniques often use physical coating processes to immobilize the polymer on the substrate surface, resulting in poor reproducibility, limited range of pH stability, and the tendency to swell when exposed to organic sorbents. Sol–gel-derived sorbents are inherently porous with their characteristic sponge-like porous architecture [5]. As such, the sample matrix can easily permeate through the micro and mesopores of the sol–gel sorbents for rapid analyte–sorbent interaction leading to fast extraction equilibrium.
Due to the open bed, planar geometry, the FPSE membrane can be used in an equilibrium-based extraction mode (as in direct immersion extraction in SPME) or in an exhaustive extraction mode (as an SPE disk). Although, the application potential of FPSE membrane as an SPE disk has not fully explored, Lakade et al. [6] has demonstrated that the FPSE membrane can be used as an SPE disk without compromising the quality of the analytical data.
Preparation of sol–gel sorbent coated FPSE membrane involves a number of decision points, including:
It is worth mentioning that steps 1–3 primarily depend on the physicochemical properties of analytes, especially the polarity and molecular state of the analytes.
Among all the microextraction techniques, FPSE is the only sample preparation technique that exploits the surface chemistry of the fabric substrate. In general, if the analytes are nonpolar, a hydrophobic substrate such as polyester is the rational choice. When the analytes are polar or medium-polar, a hydrophilic fabric substrate such as 100% cotton cellulose is the judicious selection. Sol–gel sorbents are chemically bonded to the fabric substrate. To ensure this chemical bonding, the fabric substrate should possess abundant surface hydroxyl functional groups. Another important selection criterion for the fabric substrate is its permeability so that the aqueous sample containing the analyte(s) of interest can permeate through the FPSE membrane easily even after creating the sol–gel sorbent coating on the substrate surface. The through pores of the FPSE membrane can extract the analyte(s) almost exhaustively at a short period. The selection of the fabric substrate is followed by the pretreatment of substrate to remove any residual finishing chemicals from the fabric surface. To clean the fabric substrate and to activate surface hydroxyl groups, a fabric treatment protocol has been developed. The protocol can be found elsewhere [7][8].
The most important step in preparing the FPSE membrane is the design of sol solution. The sol solution for creating sol–gel sorbent coating on the substrate surface consists of (a) one or more inorganic/organically modified sol–gel precursors, (b) a sol–gel active inorganic/organic polymer, (c) a compatible solvent system, (d) an acid catalyst, and water for hydrolysis. Among numerous available organically modified silane precursors, methyl trimethoxysilane (MTMS) is the most commonly used sol–gel precursor. Other popular sol–gel precursors include phenyl trimethoxysilane (PTMS) and 3-aminopropyl trimethoxysilane (3-APTMS).
Commercially available sol–gel active inorganic/organic polymers are abundant in number and many of them are yet to be explored as viable candidates for an FPSE sorbent. Popular polymers used in FPSE include poly(dimethyl siloxane) (PDMS), poly(ethylene glycol) (PEG), poly(tetrahydrofuran) (PTHF), and poly(dimethyl diphenyl siloxane) (PDMDPS).
Among many commercially available acid catalysts (HCl, acetic acid, hydrofluoric acid, trifluoroacetic acid, oxalic acid), trifluoroacetic acid (TFA) is the most commonly used acid catalyst in sol–gel synthesis.
The sol solution for sol–gel sorbent coating on a fabric substrate is generally prepared in an amber reaction vessel (2 oz.) by sequential addition and subsequent vortexing of the sol–gel precursor, solvent, organic/inorganic polymer, acid catalyst, and water.
A detail account on the potential chemical reactions involved in the sol–gel sorbent coating process can be found elsewhere [5].
The primary criteria for selecting the sol–gel precursor and the inorganic/organic polymer are based on the polarity and functional makeup of the target analytes. Generally speaking, the higher the number of the intermolecular interactions between the FPSE membrane and the target analytes, the higher the extraction efficiency of an FPSE membrane. The overall selectivity and extraction efficiency of a sol–gel sorbent coated FPSE membrane depend combinedly on the surface chemistry of the fabric substrate, the sol–gel precursor, and the inorganic/organic polymer. As such, the selectivity of the pristine polymers such as PDMS and PEG used in SPME and similar microextraction devices are substantially different than that of sol–gel PDMS and sol–gel PEG coated FPSE membranes. Sol–gel sorbents are highly porous and easily accessible for the aqueous/gaseous sample matrices due to their sponge-like porous 3D polymeric network.
The sol solution prepared in step 2 is employed in the sol–gel dip coating process. To initiate the coating process, a segment of the pretreated fabric is carefully submerged into the sol solution. The coating process begins as soon as the fabric substrate is introduced into the sol solution. Typically, the sol–gel coating process continues for 12 h at room temperature. Once the predetermined residence in the sol solution is over, the sol solution is discarded from the reaction vessel, and the sol–gel sorbent coated FPSE membrane is air dried for 1 h.
The air-dried sol–gel sorbent coated FPSE membrane is thermally conditioned in a special conditioning device built inside a gas chromatograph (GC) oven under continuous helium gas flow for 24 h. The temperature of the GC oven is set at 50 °C. After conditioning at 50 °C for 24 h, the FPSE membrane undergoes a cleaning protocol established to remove unbonded sol solution ingredients and reaction byproducts. The FPSE membrane cleaning protocol involves rinsing the membrane in methylene chloride: a methanol mixture (50:50 v/v) under sonication for 1 h. The rinsing solvent mixture is then drained from the rinsing vessel, the FPSE membrane is air dried for 1 h and thermally condition at 50 °C for 24 h under a helium environment. This step completes the sequence of steps involved in creating the sol–gel sorbent coated FPSE membrane. The FPSE membrane is stored in an air-tight container until it is used in fabric phase sorptive extraction.
Unlike classical microextraction techniques such as SPME, SBSE, and TFME, the membrane size in FPSE is not fixed and can be adjusted based on the analytical need. For a small volume of sample (for example, blood, plasma, saliva), a small FPSE membrane disc (1 cm diameter) can be used. For a larger sample volume (5–20 mL), a larger membrane size (e.g., 2.5 cm × 2.0 cm) is recommended. Although a larger size of an FPSE membrane favors a faster extraction equilibrium due to higher contact surface area, it requires a relatively larger volume of organic solvent for quantitative back-extraction, which may unnecessarily dilute the analytes prior to injection into the chromatographic system. It is important to note that FPSE eliminates the solvent evaporation and sample reconstitution from the sample preparation workflow, an inevitable step in the SPE workflow. As such, the volume of solvent usage in FPSE back-extraction must be kept at its lowest level as possible.
Unlike solid phase microextraction and similar sorbent based microextraction techniques, method development in fabric phase sorptive extraction is simple and straight forward. Figure 1 presents a graphical schematic of a typical FPSE workflow.
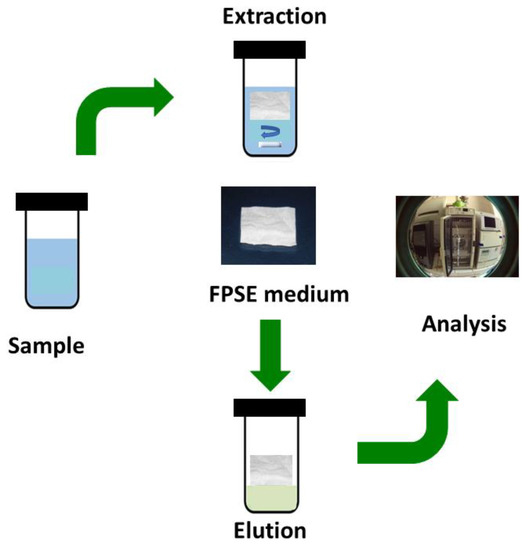
Figure 1. Typical FPSE workflow.
FPSE does not require any sample pre-treatment process to reduce/minimize matrix interferents such as filtration, protein precipitation, or centrifugation, and the FPSE membrane can be introduced directly into the sample, regardless of the complexity of the sample. However, the extraction efficiency can be substantially improved when a systematic method development strategy is followed to optimize a number of factors that directly impact on the overall extraction efficiency of the FPSE membrane. The factors are presented in Figure 2 with their relative significance. As such, an analyst may decide which factor(s) should be given more attention during the method development exercises. The factors include:
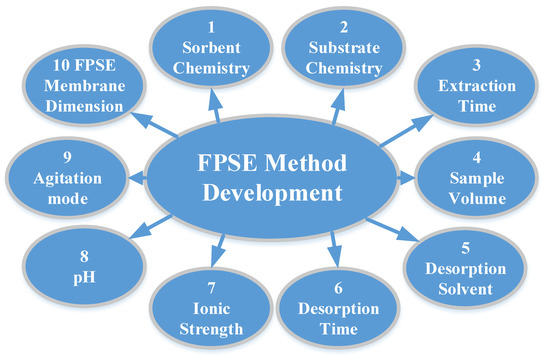
Figure 2. Factors and their relative importance on fabric phase sorptive extraction method development.
FPSE method development exercises can be carried out using a conventional one-factor-at-a-time (One FAT) approach or using a chemometric design of experiment approach. The later approach is the more scientific and green approach, as it provides deep insight about the overall extraction process and sheds light as to whether different factors interact with each other or not. A screening design can be carried out to select factors with the most influence on the overall extraction efficiency. Subsequently, a response surface model (RSM) design can be employed to find the optimum levels of the most influential factors.
As can be seen in Table 1, FPSE offers a broad range of sorbents spanning from nonpolar, to medium polar, to polar, to ionized, to mixed mode, and to zwitterionic. As such, it is practically impossible for one to determine the most efficient sorbent by real experimentation. As such, for the first time, a new approach for selecting FPSE sorbent chemistry has been developed based on an absolute recovery percentage calculator that utilizes the logKow of an analyte to predict an estimated absolute recovery of an analyte for a given FPSE sorbent chemistry. For example, the absolute recovery on sol–gel Carbowax 20M (sol-gel CW 20M) sorbent coated on 100% cotton cellulose fabric can be expressed as:
Absolute Recovery % = 4.2977487 + 22.823041 × Log Kow − 3.1343544 × (Log Kow − 2.737)2
This equation is valid for any analyte possessing a logKow value between 0.3 and 5.07, and majority of the analytes we generally encounter fall in this range. During the FPSE method development exercises, it is recommended that an analyst select the 3 best FPSE membranes, and subsequently determine the best FPSE membrane by exposing them under identical FPSE conditions. A good starting time can be:
FPSE membrane size: 2.5 cm × 2.0 cm;
Sample volume: 10 mL;
Analyte concentration: 1 µg/mL;
Extraction time: 1 h;
Stirring speed: 800 rpm;
Desorption solvent: methanol;
Desorption solvent volume: 500 µL;
Desorption time: 10 min.
The prepared sample can be injected into a gas chromatograph or high-performance liquid chromatograph to obtain the chromatographic signal area for an analyte or group of analytes.
Absolute recovery calculations for major FPSE sorbent chemistries are presented in Table 2.
Table 2. Absolute recovery calculator for selected FPSE membranes.
| Sorbent (Substrate) | Equation for Recovery% Calculation |
|---|---|
| Si-CW20M (Cellulose) | 4.2977487 + 22.823041 log Kow − 3.1343544 (log Kow − 2.737)2 |
| Si-PEG1000 (Cellulose) | −11.53483 + 20.950137 log Kow − 0.4017218 (log Kow − 2.737)2 |
| Si-PEG300 (Cellulose) | 14.758805 + 16.309632 log Kow − 5.5504622 (log Kow − 2.737)2 |
| Si-CN-CW20M (Cellulose) | −24.39275 + 23.940499 log Kow + 1.247171 (log Kow − 2.737)2 |
| Si-PPG-PEG-PPG (Cellulose) | −3.648816 + 21.546191 log Kow − 2.878525 (log Kow − 2.737)2 |
| Si-PEG-PPG-PEG (Cellulose) | −7.680093 + 23.069108 log Kow − 1.7262745 (log Kow − 2.737)2 |
| Si-PTHF (Cellulose) | 12.40054 + 17.848979 log Kow + 17.848979 (log Kow − 2.737)2 |
| Si-PTHF (Fiber Glass) | −28.44237 + 20.9507 log Kow + 3.3273496 (log Kow − 2.737)2 |
| Si-C18 (Cellulose) | −2.274875 + 20.816015 log Kow − 4.1478973 (log Kow − 2.737)2 |
| Si-C8 (Cellulose) | −3.392783 + 21.261305 log Kow − 3.7724155 (log Kow − 2.737)2 |
| Si-PDPS (Cellulose) | −10.30009 + 17.450029 log Kow − 0.2880039 (log Kow − 2.737)2 |
| Si-PDMDPS (Polyester) | −9.185327 + 17.815515 log Kow − 1.9655752 (log Kow − 2.737)2 |
| Si-PDMDPS (Cellulose) | −19.60225 + 15.453851 log Kow − 1.62186 (log Kow − 2.737)2 |
The predicted absolute recovery values often corroborate with the actual recovery values obtained from real experimentation, as demonstrated by several researchers [9][10]. It is important to note that this model was developed using analyte solution in deionized water. When the sample matrix contains too many matrix interferents, substantial deviation from the expected recovery of the analyte may be observed [11].
FPSE is the only microextraction technique that exploits the substrate surface chemistry to compliment to the overall selectivity and the extraction efficiency of an FPSE membrane. The surface property of the fabric substrate substantially impacts on the selectivity and extraction efficiency of an FPSE membrane. The dependence of the analytes’ polarity (logKow) on the nature of different fabric substrates has been estimated using a compound mixture consisting of furfural alcohol (FA, logKow 0.3), piperonal (PIP, logKow 1.05), phenol (PHE, logKow 1.5), benzodioxole (BDO, logKow 2.08), 4-nitrotoluene (4NT, logKow 2.45), 9-anthracene methanol (9AM, logKow 3.04), 1,2,45-tetramethyl benzene (TMB, logKow 4.0), triclosan (TCL, logKow 4.53), and diethylstilbestrol (DES, logKow 5.07). As can be seen from Table 3, for the sol–gel PTHF sorbent, cellulose fabric is favored for polar analytes extraction, whereas fiber glass fabric is suitable for nonpolar analyte extraction. Between sol–gel PDMDPS sorbents coated on polyester fabric and cellulose fabric, polyester is better for nonpolar analyte extraction. Since PDMDPS is a nonpolar polymer, extraction of polar analytes is not favored in either polyester fabric or in cellulose fabric. As expected, the organic/inorganic polymer plays the most significant role in the overall selectivity and extraction efficiency of FPSE membrane. However, the role of the fabric substrate cannot be ignored.
Table 3. Comparison of extraction recovery between different fabric substrates.
| Sorbent | FA (%) |
PIP (%) |
PHE (%) |
BDO (%) |
4NT (%) |
9AM (%) |
NAP (%) |
TMB (%) |
TCL (%) |
DES (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sol–gel PTHF (Cellulose) | 0 | 9.8 | 4.0 | 45.6 | 46.7 | 66.1 | 86.8 | 93.4 | 82.1 | 49.5 |
| Sol–gel PTHF (Fiber Glass) | 0 | 1.2 | 4.1 | 21.8 | 25.4 | 25.4 | 38.4 | 77.8 | 74.9 | 91.7 |
| Sol–gel PDMDPS (Polyester) | 0 | 1.7 | 0 | 13.4 | 13.7 | 67.7 | 51.8 | 83.5 | 74.9 | 46.7 |
| Sol–gel PDMDPS (Cellulose) | 0 | 0.1 | 1.8 | 9.7 | 10.0 | 11.0 | 68.7 | 50.4 | 42.1 | 68.1 |
Extraction efficiency is one of the most important factors that influence the extraction efficiency of an FPSE membrane. Generally, extraction efficiency is verified between 0 and 60 min, when most of the analytes reach the plateau of the extraction kinetic curve, and exposing the FPSE membrane longer than this time period does not yield any improvement in the extraction efficiency of an analyte in a given FPSE membrane. In some cases, when a high mass of matrix interferents are present in the sample matrix, as in the case of an environmental or biological sample, longer extraction equilibrium time may be observed.
Sample volume requirement in FPSE is flexible and depends on the availability and nature of the sample. For a smaller sample volume, a smaller FPSE membrane size can be used. If the sample is freely available, a larger FPSE membrane size (e.g., 2.5 cm × 2.0 cm) can be used, and a sample volume from 10 mL to 30 mL may be systematically investigated to determine the optimum sample volume.
Due to the strong chemical bonding between the fabric substrate and the sol–gel sorbent coating, an FPSE membrane can be exposed to any organic solvent for quantitative back-extraction of the analytes after the extraction process. As such, a single solvent or a mixture of solvents can be used to efficiently back-extract the analytes. The solvent or solvent system (mixture of multiple solvents) should be optimized to ensure quantitative back-extraction of the extracted analytes.
Since the sol–gel sorbents are inherently porous with sponge-like morphology, the diffusion of the solvent during solvent mediated back-extraction does not need any external energetic stimulus such as magnetic stirring. However, it is imperative to allow adequate time for the solvent to exhaustively scavenge the extracted analytes from the sol–gel sorbents. Most researchers have reported 5 min as the optimum desorption time, although in some cases 7.5 min or 10 min as the optimum desorption times are not unusual. For method development, a time range between 0 and 10 min can be investigated.
Ionic strength of the sample matrix can be increased by the addition of NaCl or another suitable salt to the sample to compel polar analytes out of the aqueous solution and become available for being extracted into the FPSE membrane. The optimum salt concentration can be determined by monitoring the increase in the extraction efficiency with the concentration of salt in the solution.
When acidic or basic analytes are extracted on a neutral FPSE membrane, pH adjustment of the sample matrix may be used to force the analytes to remain in their neutral state so that the neutral FPSE membrane can maximize its extraction efficiency under the given extraction conditions. It is a cumbersome process and requires obtaining an optimum matrix pH value via a series of experiments. In order to eliminate this cumbersome drill, a mixed mode sorbent coated FPSE membrane can be used.
Extraction kinetics can be expedited by applying external stimuli such as magnetic stirring, ultra-sonication, or orbital shaking during the FPSE process. The optimum stirring speed should be established experimentally during the FPSE method development.
FPSE is the only microextraction technique that allows the analyst to determine the size of the FPSE membrane. Although the typical size for a small volume of sample is a 1 cm diameter disc, or a 2.5 cm × 2.0 cm rectangular block for a larger sample volume, the analyst may use any size of the FPSE membrane depending on the analytical need.