1000/1000
Hot
Most Recent

Macrophages in cancer represent a major part of the immune cells within the TME and they are more frequently associated with a bad prognosis.
Nowadays, tumor microenvironment (TME) is recognized as an essential element of tumor development and progression. It not only remains in constant contact with the tumor, but it also mediates complex dialog between malignant cells and surrounding tissues. The cellular components of this dynamic network are represented by normal and tumoral tissue-resident cells with a large proportion of recruited immune cells alongside: fibroblasts, neuroendocrine, adipose, endothelial, and mesenchymal cells [1]. All of the cellular and molecular actors of the TME are involved in carcinogenesis through the promotion of tumor: growth, dormancy, invasion, and metastasis. The infiltrating immune cells can be represented by lymphoid cells, such as: CD8, CD4, and γδ T lymphocytes, B cells, and natural killer (NK) cells, and myeloid cells, such as: monocytes/macrophages, dendritic cells (DC), neutrophils, myeloid-derived suppressor cells (MDSC), basophils/eosinophils, and mast cells. In the initial states of oncogenesis, all of these cell populations can help in the elimination of mutated cells. However, after the tumor dormancy and editing phase, the loss of oncoantigens and MHC lead to the immune escape, allowing for further tumor development [2]. TME, including immune cells, is then modified to actively support and promote cancerogenesis and shape the character of emerging tumors [3]. Following the text, the readers can refer to the figures that resume the role of the different tumor-associated myeloid cells in cancer cells survival, proliferation, and migration (Figure 1), and in cancer cells immune-escape and therapy resistance (Figure 2).
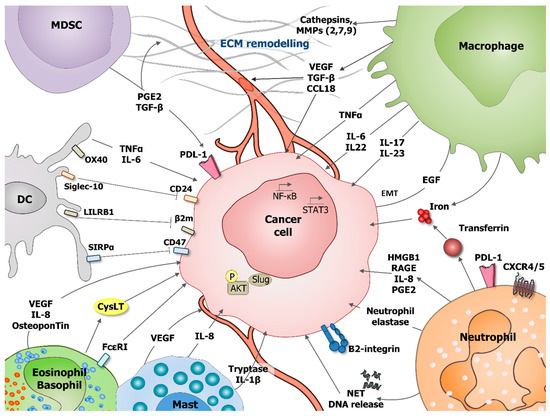
Figure 1. Role of tumor-associated myeloid cells in cancer cells survival, proliferation and migration. During tumorigenesis various myeloid cells populations, including: dendritic cells (DC), myeloid-derived suppressor cells (MDSC), macrophages, neutrophils, eosinophils, basophils, and mast cells can support cancer cells survival, proliferation, and migration. These processes can be stimulated by direct effect on tumoral cells or indirectly by influencing tumor microenvironment (TME), including extracellular matrix (ECM) remodeling and angiogenesis stimulation. Direct effects are mediated through production of interleukin IL-6, IL-8, IL-17, IL-22, IL-23, prostaglandin E2 (PGE2), transforming growth factor beta (TGF-β), vascular endothelial growth factor A (VEGF-A), osteopontin, and tumor necrosis factor α (TNF-α). Neutrophils secrete the iron-transporting protein transferrin which is a major mitogen for tumor cells and release of neutrophil extracellular traps (NET), including their deoxyribonucleic acid (DNA). Neutrophils produce neutrophil elastase favoring tumor cell proliferation and regulate the HMGB1/RAGE/IL-8 axis favoring the crosstalk between glioma cells and the TME. Mast cells release tryptase and IL-1 beta (IL1-β) mediating malignant pleural effusion. Basophils express Fcε Receptor I, promoting their tissue infiltration and producing cysteinyl leukotrienes (CysLT), allowing for proangiogenic activity of activated basophils. DC express OX40, Siglec-10, leukocyte immunoglobulin-like receptor B1 (LILRB1), and SIRPα, which, respectively, recognize OX40 ligand (OX40L), CD24, MHC class I-associated β2M subunits, and CD47 at the surface of tumor cells blocking phagocytosis. Macrophages are an important source of various metalloproteinases (MMPs, MMP2, 7, 9) and cathepsins that provide conduits for tumor cells in the extracellular matrix (ECM). VEGF that is produced by myeloid cells is a major stimulator of angiogenesis.
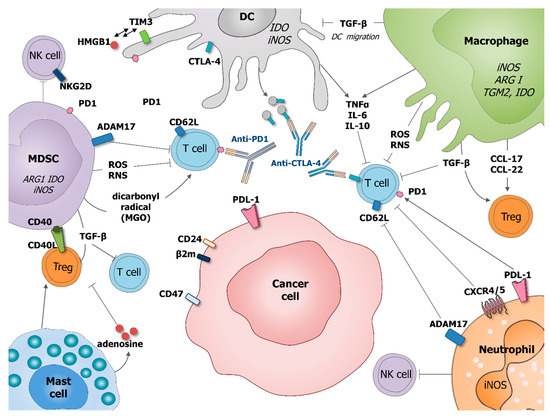
Figure 2. Role of tumor-associated myeloid cells in cancer cells immune-escape and therapy resistance. Macrophages and MDSC produce transforming growth factor beta (TGF-β) which inhibits DC migration at the tumor site, promote regulatory T cells (Treg) and block T cell activation. Macrophages potentiate Treg activation by production of chemokines CCL-17 and CCL-22. DC express immune checkpoint receptors, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which can be released on the surface of microvesicles that could block costimulatory molecules, such as CD80/86. DC express also programmed cell death protein 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) interacting with HMGB1. MDSC suppress T cell functions by producing ROS and RNS inducing the nitration of TCR and MHC-I, as well as producing dicarbonyl radical methylglyoxal in the TME inhibiting CD8 T cells. MSDC express CD40 interacting with is ligand CD40L present on the surface of Treg. Mast cells can stimulate Treg numbers and secrete adenosine, which inhibits T cell proliferation. Neutrophil, as MDSC, expresses a disintegrin and metalloproteinase 17 (ADAM17) that cleaves the ectodomain of L-selectin (CD62L) on T cells. Neutrophil and cancer cells might express PD1 ligand (PDL-1) which inhibits activation of T cells expressing PD1. Neutrophils express CRCR4/5 leading to the immunosuppression of T-cell proliferation. MDSC and neutrophils, are also able to suppress NK cell cytotoxicity. By diminishing the response of various immune cells, tumor-associated myeloid cells can also negatively influence outcome of anti-cancer therapies, especially various immunotherapies.
It was recently shown that most resident macrophages (MPs) in normal tissues are not only derived from bone marrow (BM) progenitors, as previously thought, but also from yolk sac or foetal liver and they are maintained by self-renewal [4][5]. However, during adult life, the rate of resident MPs can also be maintained by the infiltration of blood-derived MPs, except for microglia in the brain [6]. Therefore, monocytes from blood and bone marrow are able to infiltrate tissues and differentiate into specific tissue MPs, but whether they can be considered to be tissue-resident MPs is still a debate. In the blood, two types of monocytes can be distinguished by the expression of the Ly6C marker in mice, Ly6C+ monocytes being originate from bone-marrow, and Ly6C− from circulating Ly6C+ monocytes [7]. If Ly6C+ monocytes respond to damage by infiltrating and differentiating in MPs in the tissues, Ly6C− monocytes remain in the vessels to detect and remove damaged endothelial cells [8]. Therefore, the ratio of these two monocytes populations in the blood can change, depending on different stimuli, including external stimuli. In human blood, three main monocytes populations have been identified, a classical one, CD14++CD16−, a non-classical one, CD14intCD16+, and an intermediate one, CD14+CD16+ [9]. These two latter populations are differentiated from a uniform population CD14++CD16− egressing from the bone marrow [10].
Resident macrophages display key roles in growth, tissue development, homeostasis, and remodelling, and they have site-specific phenotypes and functions [11]. It was proposed that the specialization of the resident MPs takes place inside the target tissue, due to close contacts with tissue-specific cells as well as to soluble factors in the tissue environment [12]. Microglia, in the brain and spinal cord, contribute to synaptic maturation during brain development and the clearance of immature or defective neuronal synapses [13]. In the lung, alveolar MPs mediate approximately 30% of the surfactant lipid metabolism. Langerhans cells with cutaneous MPs in the skin are specialized in the formation of the extracellular matrix and in skin layer differentiation. Cardiac-resident MPs are required during heart development and they take part in the regulation of the cardiac rhythm [14]. Kupffer cells in the liver are involved in the modulation of metabolism in hepatocytes, preventing the pathogenic accumulation of lipids [15]. Tissue-resident MPs that are located in the red-pulp region of the spleen have important functions in iron processing connected with the clearance of damaged red blood cells and the erythropoiesis [16]. Besides, MPs from the white-pulp region phagocytose lymphocytes avoiding B cell accumulation and auto antibody production [17].
Apart from physiological functions, monocytes/MPs also display pathological functions in infection/inflammation contexts, tissue repair, and cancer.
MPs are very plastic cells that are able to respond to molecular or cellular signals from the tissue environment. The molecular signals can be endocrine or paracrine signals that originate from phagocytosed cells or microorganisms and from the extracellular matrix/proteins. MPs can also directly interact with other tissue-resident cells, such as immune cells recruited during injury. Indeed, monocytes and MPs are recruited from the bone marrow to the tissue injury site via the chemoattractant CCL2 that is secreted by resident MPs, endothelial cells, myocytes, and fibroblasts [18]. The CCL2 receptor, CCR2, is highly expressed by Ly6C+ mouse monocytes [19]. In humans, classical monocytes (CD14+CD16−) display a high expression of CCR2 and they are involved in responses to bacterial infection and inflammation, in inflammasome signalling, and in low density lipoprotein uptake. In contrast, non-classical monocytes (CD14intCD16+) display a high expression of genes that are involved in cytoskeletal dynamics, tissue invasion during inflammation and genes suggesting terminal differentiation and cellular maturity [20].
Therefore, monocytes-derived and tissue-resident MPs colocalize to take part in healing and then to the resolution, thanks to the production of cytotoxic and pro-inflammatory mediators, the clearance of invading microorganisms, or removal of apoptotic and damaged cells [21]. On the arrival at the injury site, blood-derived MPs can adopt a pro-inflammatory/M1/classical or anti-inflammatory/M2/alternative phenotype, depending on the cytokines that are present in the microenvironment [22][23]. Regarding the plasticity of MPs, although the framework of M1/M2 polarization is a very useful system for in vitro studies, it is unclear how similar clear-cut phenotypes can be appended during in vivo injury and repair [24]. This M1/M2 paradigm is well pictured by the high expression of M2 markers by tissue-resident MPs when compared to the mature phenotype of monocyte-derived MPs [25][26]. Early on after damage or injury, infiltrated Ly6C+ monocytes scavenge apoptotic debris or pathogens or infected cells thanks to the expression of pattern recognition receptors (PRR), Toll like receptors (TLR), scavenger receptors, and Fc receptors that, respectively, recognize microbial antigens, danger signals, or immunoglobulins. These events lead to the activation of transcription factors such as interferon (IFN) regulatory factors and nuclear factor kappa B (NFκB), inducing an M1 polarization and initiation of the inflammatory response [27]. These blood-derived M1 MPs then release pro-inflammatory cytokines (e.g., IL-1β, IL-6, IL-12, IL-23, and TNF-α) and type-1 cell-attracting chemokines (e.g., CXCL9 and CXCL10), favouring the recruitment of more macrophages and leucocytes to help with injury resolution [28]. Once the acute injury has been resolved, MPs are in charge of suppressing inflammation and initiating wound repair. After clearing debris, MPs produce growth factors and mediators, which abrogate the pro-inflammatory function of T cells and other immune cells [29]. This is accompanied by a progressive repolarization of the blood-derived MPs towards a phenotype and functions that are increasingly similar to those of homeostatic tissue-resident MPs [30].
Specific plasma membrane receptors induce pro- and anti-inflammatory pathways in MPs. If IFNγ mediates the classical/M1 activation with upregulation of MHCII antigens, induction of nitric oxide synthase (i-NOS) and the production of pro-inflammatory molecules, interleukin-4 and -13 (IL-4 and IL-13) induce the alternative/M2 phenotype that is characterized by the upregulation of CD206, transglutaminase 2, arginase, and the production of IL-10 and chemokines, such as CCL17, CCL22 and CCL24 [28][31]. Like other immune cells, specific functions of MPs are, therefore, coupled to specific phenotypes, even when considering their plasticity, MPs can display intermediate phenotypes in certain inflammatory diseases and cancer [32][33].
MPs in cancer are called tumor associated macrophages (TAM) and they represent the major immune component of the TME. According to oxygen ratio and tumor progression, TAM display either a M1 or M2 phenotype. They play a major role in tumor growth, metastatic dissemination, and therapy failure, promoting angiogenesis and secreting different factors that are involved in extracellular matrix (ECM) remodelling that facilitate tumor cell motility and intravasation. High TAM infiltration is generally correlated with poor outcomes in several types of cancer.
Until recently, TAM were considered to exclusively originate from blood-derived MPs undergoing differentiation upon tissue infiltration in response to chemokine and growth factors that are produced by stromal and tumor cells in the TME. Colony-stimulating factor 1 (CSF-1), vascular endothelial growth factor A (VEGF-A), and different CCL (2, 18, 20) were found to act as chemotactic molecules in various cancer [34][35][36]. However, evidence shows that tissue-resident MPs can coexist in tumors with blood-derived MPs and their phenotype can rapidly evolve, depending on the stage of the tumor and the characteristics of the molecular and cellular actors in the TME.
In early stage tumor development, IFN-α polarizes resident MPs towards an M1 phenotype and activates the infiltration of blood derived-M1 MPs. These MPs directly phagocytize tumor cells expressing low levels of the “don’t eat me” signal CD47, release pro-inflammatory factors that activate Th1 and Th17 immune responses and can also produce TNF-related apoptosis-inducing ligand (TRAIL) that result in TRAIL-induced cancer cell apoptosis [37][38]. In contrast, in more advanced tumors, TAM are polarized to an M2 related phenotype. This polarization occurs, thanks to anti-inflammatory mediators that are produced by the tumor cell itself and by stromal and immune cells in the TME, but also by MPs themselves. CSF-1, CCL2, 3, 14, and IL-4 are common tumor-derived factors driving the recruitment, proliferation, and M2-polarization of MPs [39][40][41]. Other factors are more specific to the type of cancer, such as prostate cancer-derived cathelicidin-related antimicrobial peptide [42] or hypoxic cancer cell-derived cytokines Oncostatin M and Eotaxin [43]. IL-4 can also be secreted in the TME by Th2-polarized CD4 cells as well as IL-10 or IL-13, which lead to STAT-6 activation [44][45][46]. Besides, migration-stimulating factor (MSF), IL-4, and CXCL12 can be secreted by MPs to promote self-polarization [47][48][49]. Finally, hypoxia-inducible factors (HIF-1α and 2α), high-mobility group box 1 protein (HMGB1), extracellular ATP, or tumor-derived ECM components are also potential factors that promote M2 polarization [50][51][52].
M2-like MPs in TME are highly involved in cancer cell resistance by promoting cancer initiation, angiogenesis, the establishment of a premalignant niche, metastasis, and immune suppression.
It has been shown that an inflammatory microenvironment promotes genetic instability, leading to the proliferation of epithelial cells, but also the infiltration of immune cells, such as macrophages. On site, TAM can secrete IL-23 and IL-17, which promote cancer cell proliferation. IL-23 signaling in tumor cells is important for the intra-tumoral production of downstream cytokines, which are either direct (IL-6, IL-22) or indirect (IL-17A) STAT3 activators [53]. IL-6 that is produced by TAM also promotes tumor cells proliferation and invasive potential via STAT3 signaling [54]. TAMs also represent a strong source of iron, which is essential in tumor cell division, growth, and survival, and motility through the remodeling of the extracellular matrix [55].
TNF-α, which is a key player in NFκB upregulation, is produced in the TME by, amongst others, TAM and it induces migration and invasion potential of cancer cells [56]. Cancer cells motility is also favored by various metalloproteinases (MMPs) and cathepsins that are produced by TAM activated by TGF-β in the TME [57][58]. Some chemokines, such as CCL18 produced by TAM, also promote the migration of cancer cells and metastasis through the clusterization of integrins [59]. A mouse study showed that mouse MPs-derived insulin growth factor 1 (IGF-1) induces the migration of epithelial ovarian cell lines [60]. Finally, it has been shown that MPs-derived microRNA (miR-223) also regulate tumor invasion [61].
TAM also take part in the promotion of the formation of blood vessels within the tumor providing nutrition for tumor growth [62]. Several pro-angiogenic factors, such as TGF-β, VEGF, PDGF, and angiogenic chemokines, are produced by TAM in the TME. CCL18 produced by TAM promote, synergically with VEGF, the endothelial cell migration and angiogenesis [63]. Other chemokines, such as CXCL1, 8, 12, 13, and CCL2, 5 produced by TAM, help with the angiogenesis switch in tumor tissues [28]. Finally, TAM can be found in hypoxic parts of the tumor and it can express HIF-1α, which regulates the transcription of VEGF largely associated with angiogenesis [58].
The epithelial-mesenchymal transition (EMT) is a fundamental process for tumor progression and metastasis, during which TAM plays an active role though interactions with tumor cells and the production of facilitating factors of EMT. Through the production of EGF, TAM can induce the EMT of cancer cells by activating the EGFR/ERK1/2 signaling pathway [64].
TAM are also able to protect tumor cells from immune attacks, inhibiting T cell proliferation, function, and recruitment through the release of immunosuppressive cytokines. They are able to neutralize the recruitment and functions of cytotoxic CD8 T-cells and natural killer cells through the secretion of IL-10 and TGF-β in the TME [65][66]. TAM-derived TGF-β also decreases antigen presentation, which reduces DC migration and increases apoptosis [67]. On the contrary, the production of CCL17 and CCL22 by TAM promotes the infiltration of Th2 and Treg populations in tumors [68]. TAM-derived prostaglandin E2 (PGE2), IL-10, and indoleamine 2,3-dioxygenase (IDO) play important roles in the suppression of cytolytic T lymphocytes and in the induction of Treg function [69][70]. IL-10, alone or in concert with IL-6, causes the upregulation of macrophage B7-H4 expression, which is responsible for the suppression of tumor-associated antigen-specific T cell immunity [71]. Moreover, IL-10 and PGE2 can induce the expression of immune-checkpoint ligands (PD-L1) in myeloid cells, which can inhibit cytolytic T lymphocytes responses [72][73]. Finally, IL-10 acts in an autocrine circuit in TAM in order to restrain their expression of IL-12 and also inhibits the release of IFN-γ [48].
The resistance of tumor cells to cytotoxic T cells can also be induced by reactive oxygen species (ROS) and reactive nitrogen species (RNS) that are produced by TAM through iNOS and arginase I, two enzymes that are very active in TAM [74].
TAM can also be responsible for tumor resistance to treatments. TAM-derived exosomes have been shown to be involved in mediating the resistance of gastric cancer cells to cisplatin [74]. Endocrine resistance in breast cancer cells can be increased by TAM-derived CCL2 through the activation of the PI3K/Akt/mTOR signaling pathway [40]. Autophagy in hepatocellular carcinomas cells can be induced by TAM, leading to oxaliplatin resistance [75]. It was recently shown that depletion of TAM by an anti-CSF-1R enhanced the anti-tumor effect of docetaxel in a murine epithelial ovarian cancer [76]. In Chronic Lymphocytic Leukemia (CLL), the nurse like cells (NLC), which are the specific protumoral TAM of the CLL reside in lymph nodes, spleen and bone marrow where they protect CLL B cells against apoptosis but also against chemotherapies such as ibrutinib [77][78]. This protection has been shown to depend on cell contact and soluble factors that are produced by TAM and, in an autocrine manner, by CLL B cells themselves [79][80][81][82].
Anticancer immunotherapies may also be reduced by TAM when their suppression of TME correlates with an increase of DC-vaccination therapy in a malignant mesothelioma mouse model [83] or an increase of anti-PD1 treatment favoring CD8 T cells recruitment to the tumor site [84][85]. Finally, high levels of TAM in the TME were also shown to increase the resistance of tumor cells to Vascular-targeted photodynamic: paldeliporfin (VTP) therapy in a prostate mouse model [86].