1000/1000
Hot
Most Recent

Grapes are rich in primary and secondary metabolites. Among the secondary metabolites, polyphenolic compounds are the most abundant in grape berries. Besides their important impacts on grape and wine quality, this class of compounds has beneficial effects on human health. Due to their antioxidant activity, polyphenols and phenolic acids can act as anti-inflammatory and anticancerogenic agents, and can modulate the immune system. In grape berries, polyphenols and phenolic acids can be located in the pericarp and seeds, but distribution differs considerably among these tissues. Although some classes of polyphenols and phenolic acids are under strict genetic control, the final content is highly influenced by environmental factors, such as climate, soil, vineyard, and management.
In 2017 the worldwide grape production was 73 million tons and wine consumption was 244 hectolitres, which represents the grapevine as one of the main horticultural crops in the world [1]. The grapevine is part of the large genus Vitis which consists of 70 species divided into two subgenera, Muscadinia and Euvitis. Based on the geographical origin, Euvitis group can be divided into European (V. vinifera L.), East Asian (V. amurensis Rupr.), and North American species (V. labrusca L., V. riparia Michx., V. berlandieri Planch., etc.). One more group that can be considered as a specific group of grape cultivars includes interspecific hybrids, made by crossing V. vinifera cultivars with cultivars belonging to other Vitis species. In recent years there has been a growing interest in studying these cultivars [2][3][4][5] because of their quite different polyphenolic content than V. vinifera cultivars. Furthermore, they possess a level of resistance to some fungal diseases and pests.
Vitis vinifera has a long domestication history, and during the long cultivation period, thousands of grape varieties were developed, many of which are still in production [6]. Grapes are rich in primary and secondary metabolites which affect the quality. There are three distinct tissues in a grape berry (skin, pulp, seeds) which contain different groups of compounds, such as organic acids, sugars, volatile compounds, polyphenols, and phenolic acids. Polyphenolic compounds, together with phenolic acids, are a large group of plant secondary metabolites that can be divided based on structure. Polyphenols are defined as substances that possess multiple aromatic rings with one or more hydroxyl groups, while a phenol is any compound that contains an aromatic ring—regardless of the number of them—with one or more hydroxyl groups attached. Such a definition is not appropriate when referring to plant phenols because it would also include estrone and some carotenoids that are terpene by origin. In general, plant phenols and polyphenols refer to natural secondary metabolites derived from the shikimate/phenylpropane pathway and/or polyketide acetate/malonate pathway to form monomeric or polymeric forms, as chemically defined, and they are included in a very large number of physiological processes in plants [7][8]. According to this definition, stilbene and flavonoids are polyphenols, while phenolic acids are not polyphenols, so the terms phenols and phenolic compounds will be used hereinafter when phenolic acids, stilbene, and flavonoids need to be considered. One of the largest polyphenolic groups present in grapes is the flavonoids, which include anthocyanins, flavonols, and flavan-3-ols. Furthermore, grapes contain phenolic acids (hydroxycinnamic and hydroxybenzoic acids) and stilbenes. All these compounds play important roles in growth, reproduction, and defense reactions in plants. Based on the presence or absence of phenolic compounds, grape varieties can be divided into red and white varieties. Furthermore, these compounds have considerable influences on grape/wine quality and sensory characteristics—particularly astringency, bitterness, and color stability [9][10]. The final content and composition of phenolic compounds are influenced by multiple factors, such as grape variety, climate, soil, and growing conditions [11].
In the 1990s the phrase "French paradox" was established for observations that the French population suffered a lower incidence of coronary heart disease (CHD) despite the high intake of saturated fat. This paradox was attributed to high wine consumption [12]. Since then, many studies have focused on the benefits of moderate wine consumption and grape and wine compounds that have beneficial effects on human health [13][14][15][16][17]. It was shown that phenols have antioxidant activity, can be anti-inflammatory, anticancerogenic, and antibacterial, and can modulate the immune system. Hence, there is a growing interest in using these compounds in the food industry as natural additives, food coloring agents, or seasonings [18].
In the last ten years, numerous review papers and chapters have been published that provide overviews of the chemistry and biochemistry of polyphenols [11][19][20][21][22], their composition and content in grapes [23], their impacts on human health [24][25], and their interactions with different aspects of human health in more detail, such as anticancer [26], neuroprotective [26][27][28][29][30][31], anti-inflammatory [32][33][34], cardiovascular [35], and anti-diabetic [36] actions.
The analysis of phenolic compounds consists of collecting the samples, sample preparation, instrumental analysis, and data processing. The most important step, which will affect the instrumental analysis and the data obtained, is sample preparation. This is the most critical and demanding step to improve the analysis concerning the matrix, the analyte, or both. Thus, the optimization of sample preparation reduces the total analysis time and avoids potential error sources, especially when working with samples at low concentrations [37].
A major step in sample preparation is the extraction process, including the choice of extraction technique, which is important for achieving good recoveries [8]. There are many extraction techniques for the recovery of phenols from grapes, such as solid–liquid extraction (SLE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE), matrix solid-phase dispersion (MSPD), supercritical fluid extraction (SFE), and pressurized liquid extraction (PLE). Details about above-mentioned techniques for extraction of phenolic compounds can be found elsewhere [21].
Grapes and leaves are a rich source of phenolic compounds, and in the grape berry, they can be found in the skin, pulp, and seed. These different tissues of the grape berry have different contents and compositions of phenolic compounds. The skin of a grape berry contains tannins and pigments, the pulp contains juice but no pigments, and seeds contain tannins. Biosynthesis of all phenolic compounds goes through the phenylpropanoid pathway from the amino acid phenylalanine, and two classes of compounds can be produced, flavonoids and stilbenes [11][38]. Phenolic compounds, as secondary metabolites, are frequently accumulated as glycosides. Thus, nonflavanoids accumulate in the vacuoles of mesocarp cells while flavonoids accumulate in the dermal cells of the skin tissue [39]. Many factors influence the biosynthesis of phenolic compounds, among which the most important is the genotype (cultivar). Other factors are related to environmental conditions in which the cultivar is grown, especially light, temperature, soil, and water availability. Additionally, the different management practices, such as irrigation, fertilization, yield management, and canopy management, can also have considerable influences on grape phenolic composition [40]. One more factor that has a considerable influence on determining the content of phenols in grapes is the analysis procedure, especially the extraction method used. Over the years many publications related to the analysis of grape and wine phenolic compounds have been published. Nonetheless, there is still no available standardized procedure for sample preparation, extraction, and analysis [15]. Furthermore, the content of phenolic compounds is expressed in different ways, which hinders the comparison of results.
There are many review papers and book chapters which describe the biosynthesis of phenolic compounds in grapes [22]. In brief, the biosynthesis of phenolic compounds can be divided into several interconnected pathways. The first pathway, the phenylpropanoid pathway, includes the conversion of phenylalanine by three successive enzymatic reactions to 4-coumaroyl-CoA. The above-mentioned enzyme reaction is catalyzed by the following enzymes: phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxyliase (C4H), and 4-coumaroyl: CoA ligase (4CL). Phenolic acids are the products of modifications of intermediates of the phenylpropanoid pathway, and 4-coumaroyl-CoA, the end product of this branch, can be converted to an intermediate (tetrahydroxychalcone) for flavonoids' biosynthesis by the action of chalcone synthase (CHS) or by stilbene synthase (STS) to an intermediate for stilbene biosynthesis of resveratrol (Figure 1) [41].
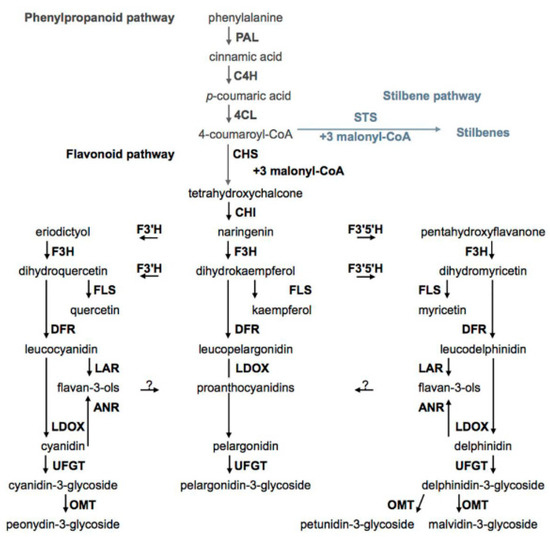
Figure 1. Biosyntheses of phenolic compounds. PAL—phenylalanine ammonia-lyase, C4H—cinnamate-4-hydroxyliase, 4CL—4-coumaroyl:CoA ligase, STS—stilbene synthase, CHS—chalcone synthase, CHI—chalcone isomerase, F3'H—flavonoid-3′-hydroxylase, F3′5′H—flavonoid-3′,5′-hydroxylase, F3H—flavanone-3-hydroxylase, FLS—flavonol synthase, DFR—dihydroflavonol reductase, LAR—leucoanthocyanidin reductase, LDOX—leucoanthocyanidin dioxygenase, ANR—anthocyanidin reductase, UFGT—UDP-glucose:flavonoid-3-O-glycosyl transferase, OMT—anthocyanin O-methyltransferase.
The second pathway is the flavonoid pathway. By this pathway biosyntheses of flavonols, flavan-3-ols, proanthocyanidins, and anthocyanidins occur. Chalcone isomerase (CHS) converts tetrahydroxychalcone to flavanone naringenin. Other flavanone eriodictyol and pentahydroxyflavanone are products of conversion of naringenin by enzymes flavonoid-3′-hydroxylase (F3′H) and flavonoid-3′,5′-hydroxylase (F3′5′H), respectively. By the activity of flavanone-3-hydroxylase (F3H), naringenin, eriodictyol, and pentahydroxyflavanone give dihydroxyflavonols, dihydroxykaempferol, dihydromyricetin, and dihydroquercetin, respectively. Enzyme flavonol synthase (FLS) catalyzes the conversion of three dihydroxyflavonols to the corresponding flavonols. The dihydroxyflavonols are also intermediates in the biosynthesis of flavan-3-ols and anthocyanins. In this branch of flavonoids biosynthesis, they are first converted to corresponding leucoanthocyanidines by the action of dihydroflavonol reductase (DFR). Leucoanthocyanidin reductase (LAR) converts leucoanthocyanidines to flavan-3-ols, and leucoanthocyanidin dioxygenase (LDOX) catalyzes the biosynthesis of the corresponding anthocyanidins. Anthocyanidins and flavonols are present in form of glycosides and this reaction is catalyzed by UDP-glucose: flavonoid-3-O-glycosyl transferase (UFGT) [11][22].
In the last few decades, there has been a growing interest in establishing a healthy diet and lifestyle that will maintain overall health and prevent stress-related diseases, such as cardiovascular disease (CVD), cancer, and diabetes [35]. Wine, grapes, and grape products have been consumed since ancient times. Grapes contain various nutrient elements, such as minerals, vitamins, edible fibers, and phytochemicals, among which the most important are polyphenolic compounds [24]. A lot of positive properties are attributed to phenolic compounds, such as antioxidant, anti-inflammatory, anticancerogenic, and antibacterial activity.
Oxidative stress is an imbalance between antioxidant molecule production and the oxidative reactive species. This delicate balance aims to maintain a suitable redox potential. If the redox potential increases, there is a risk of disease occurrence, such as cardiovascular, metabolic, and neurodegenerative diseases. Thus, research in dietary intake that may reduce the incidence of these chronic diseases has become important [42].
Phenolic compounds can act as antioxidants mainly due to their redox potential, which allows them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers [43]. Oxidation of lipoproteins, such as LDL, is an important step in the development of atherosclerosis. Therefore, dietary supplementation with antioxidant preparations containing polyphenols may reduce the risk of atherosclerosis [44]. In a study on healthy and hemodialysis subjects, the effect of concentrated red grape juice was studied. It was shown that grape juice consumption increased the antioxidant capacity of plasma, and reduced the concentration of oxidized LDL [45]. Leong et al. [46] studied the effect of Pinot noir juices on the protection of human intestinal Caco-2 cells from H2O2-induced stress. It was observed that the cell viability of Caco-2 under oxidative stress is positively correlated with the content malvidin-3-O-glucoside and total phenolics. Similar research was conducted by Wang et al. [47], wherein grape phenolic extract (GPE), rich in hydroxybenzoic acids, flavonols, and hydroxycinnamic acids, was used to observe the antioxidant protection of Caco-2 cells. The results showed that treatment with GPE was able to considerably reduce oxidative and protein damage, and reduce pro-oxidant-cytotoxicity. A study by Lingua et al. [48] on Caco-2 cells evaluated the effect of gastrointestinal (GI) digestion on antioxidant activity (AC) of white grapes, comparing them to those of their white wines. It was shown that the winemaking process modified the phenolic profile of grapes and on average only 12% of the TP (total polyphenols) content in grapes was present in wines. Furthermore, digestion reduced the phenolic content, with only 31% and 67% of native polyphenols from grapes and wines being potentially bioaccessible. It was also shown that cellular AC of non-digested and digested food was the same at the same polyphenol concentration. This indicates that changes in phenolic profile did not modify the bioactivity.
A high-fat-diet (HFD) in mice can induce leaky gut syndrome combined with low-grade inflammation, which can increase reactive oxygen species (ROS) in the intestines and may contribute to dysbiosis and metabolic syndrome (MetS). A study on mice fed a HFD were administered GPE and β-carotene [49]. The results showed that GPE, rich in proanthocyanidins (PCA), decreased HFD-induced ROS. This reduction of ROS may benefit anaerobic and microaerophilic gut bacteria, such as Akkermansia muciniphila. This implies that the bloom of beneficial gut bacteria associated with improved metabolic status may be linked to the protective antioxidant activity of polyphenolic compounds. The influences of grape skin polyphenols may differ depending on their concentrations and intracellular ROS. In research on human intestinal cells (HT-29), in basal and stressed conditions, polyphenols showed pro-oxidant and anti-oxidant effects. In basal conditions, the pro-oxidant effect, corresponding to high ROS and low reduced glutathione (GSH) content, was due to the polyphenolic oxidation in cell culture with the production of hydrogen peroxide. On the other hand, in stressed conditions grape skin polyphenols showed anti-oxidant effects up to 1.3 × 10−6 µg/g and restored the stress-related GSH reduction [50].
Grape pomace, containing skins, seeds, and stems, is considered a waste in the winemaking process, and is usually discarded, despite its high content of bioactive compounds, including polyphenols. However, with the growing interest in sustainable winemaking, there is an awareness of using this waste and its bioactive compounds for different purposes, such as food coloring agents, nutritional additives, or as agents in pharmaceutical formulations. Cristea et al. [51] proposed using the grape marc extract for the development of a new food dye with antioxidant properties of natural origin. Grape pomace (GP) obtained from Nero d'Avola grape, rich in polyunsaturated fatty acids and polyphenols, presents strong antiradical and antiproliferative activity. The incubation of human hepatoma cell lines Hep-G2 with polyphenols showed a considerable effect on cell inhibition. The antioxidant activity was shown on fibroblasts HS-68, exposed to inducers oxidative stress. The incubation of cells for 24 h with polyphenols considerably inhibited cytotoxicity of the pro-oxidants [52]. Besides the grape pomace, the leaves can also be used as a source of many polyphenolic compounds. Maia et al. [53] showed that Pinot noir leaves are rich in phenolic compounds with antioxidant activity, such as caffeic acid, kaempferol, quercetin. All that should be very interesting for the pharmaceutical and food industries.
Inflammation is a protective response of a tissue against cell injury, irritation, or pathogen invasion, and several environmental stress factors may cause inflammation [54]. The mechanism of proanthocyanidin's (PAs) anti-inflammatory activity was investigated. Li et al. [55] evaluated grape seed proanthocyanidin's anti-inflammatory activity in vitro and in vivo on rats and mice. It was observed that PAs had an inhibitory effect on paw edema in rats and ear swelling in mice. The author proposed that the major anti-inflammatory mechanism of PAs is related to its suppressive effect on the formation of inflammatory cytokines. Similar research was conducted on diet-induced obesity rats. The results showed that the daily consumption of PAs prevents inflammation in the adipose tissue, muscle, and liver, which might improve obesity-induced insulin resistance in these tissues [56]. Stilbenes are another class of polyphenols that exhibit anti-inflammatory activity, and plants containing stilbenoids have been used extensively in folk medicine [57]. Piceatannol is a stilbene compound that can be found in relatively low concentrations in wine but has strong antioxidant and anti-inflammatory activity. This was shown in a study where piceatannol inhibited mast cell-mediated allergic inflammatory reactions and their possible mechanisms, such as histamine release and MAPK pro-inflammatory cytokines. Resveratrol, the most known stilbene compound, also showed anti-inflammatory activity. It can have a protective effect in acute colitis, an inflammatory disorder, by reducing PGD2 production and the overexpression of COX-2. Additionally, it caused a considerable increase in TNBS-induced apoptosis [58]. Furthermore, resveratrol can suppress apoptosis and inflammatory signaling through its actions on the nuclear factor kappaB (NF-ĸB) pathway in human chondrocytes. These results suggest that resveratrol should be considered for the prophylactic treatment of osteoarthritis in human and companion animals [59].
CVD is a major health problem worldwide affecting considerable proportions of the populations of developed countries. CVD is associated with high cholesterol levels in the blood—in particular, low-density lipoproteins (LDL). It is widely accepted that an excessive dietary intake of saturated fats and an unhealthy diet increases cholesterol and LDL levels in the blood [15][60][61] Thus, the research by Renaud et al. [12] that the French population suffered a relatively low incidence of coronary heart disease (CHD) despite the high intake of saturated fat, has sparked the interest of many researchers. The so-called "French paradox" was attributed to moderate wine consumption and its polyphenolic compounds. In a study on 69 male and female subjects, the effects of red wine and red grape extracts were studied. It was observed that moderate red wine consumption increased HDL-C by 11–16% and decreased fibrinogen by 8–15% compared with drinking water. Additionally, the red wine group had a mean weight loss of 0.11 kg. This suggests that moderate red wine consumption is associated with beneficial changes in blood lipids and fibrinogen that may reduce the CV risk factors [62]. Furthermore, alcohol, red wine, and polyphenolic grape extracts may inhibit platelet adhesion to fibrinogen which could attribute to the cardioprotective effects of prolonged and moderate alcohol or red wine consumption [63]. Consumption of capsules with polyphenolic extracts, containing 800 mg of polyphenolic compounds, lowers systolic blood pressure by 3 mmHg and diastolic blood pressure by 3 mmHg. Catechins and procyanidins are likely the class of flavonoids contributing to this blood pressure-lowering effect [64]. Chaves et al. [65] showed that acute consumption of grape extracts (GPE), equivalent to 1.25 cups of fresh grapes, considerably improved brachial artery flow-mediated dilation (FMD) within 3 h of consumption. Further GPE consumption twice a day for 3 weeks further improved FMD, and total antioxidant capacity in plasma was slightly increased. Additionally, the GPE consumption concomitant with high-fat meal prevented high fat-induced vascular endothelial dysfunction. A study conducted on rats during 14 months studied the long term impact of phenolic compounds (PC) on age-associated cardiac remodeling [66]. The three-month-old rats were daily treated till they were middle-aged with different doses of PC. It was observed that long-term daily consumption of PC preserves cardiac morphology and performance with less hypertrophy, inflammation, fibrosis, and cardiomyocyte apoptosis. These results showed that daily consumption of PC could have a positive effect on heart protection during aging. Poti et al. [67] performed a meta-analysis on data extracted from randomized controlled clinical trials involving subjects taking polyphenol-based supplements. The authors included 34 studies and performed a meta-analysis of the main parameters evaluated in the selected studies. The analyzed data showed high heterogeneity because of the differences in the treatment, in terms of formulation, dose, source, and identity of the evaluated polyphenol. Despite that, the overall analysis revealed a considerable effect of polyphenols in positively modulating the cardiovascular parameters considered. The polyphenols positively affected blood pressure, lowering systolic and diastolic pressure. The treatments lowered plasma levels of LDL-C while increasing HDL-C levels and FMD percentage. No considerable effects were detected for high-sensitivity C-reactive protein (hs-CRP). The authors concluded that even though these effects are statistically considerable, the detected differences are of modest size and their clinical benefits need further research.
Phenols possess multiple neuroprotective effects that include protecting neurons from damage caused by neurotoxins, possessing the ability to prevent neuronal inflammation, and improving memory, learning, and cognitive functions. These effects can be achieved through two mechanisms. Phenols interact with important signaling pathways in the brain, causing inhibition of neurotoxin-induced apoptosis and promoting neuronal survival and differentiation. This group of compounds selectively acts on many protein kinases and the mode of signal transduction that regulate certain transcription factors responsible for the expression of certain genes. Polyphenols have a positive effect on the vascular system and thus affect the cerebrovascular circulation, which ultimately results in angiogenesis, neurogenesis, and changes in the morphology of neurons, thereby improving the ability to remember and learn. The formation of accumulations of amyloid fibrils is a common feature of many neurological diseases, such as Alzheimer's (AD), Parkinson's (PD), Huntington's (HD), and prion diseases. The formation of amyloid fibrils is due to aggregation and deposition of misstructured proteins resulting from errors in axon signaling, inhibition of proteasomal activity, errors in DNA transcription, and increased levels of oxidative stress, which ultimately results in neuronal dysfunction. Phenols inhibit the formation of amyloid fibrils through special aromatic interactions, thereby preventing the formation of structured fibrils with a cytotoxic effect [68].
In the past few years interest in the concept and practice of chemoprevention as an approach to control cancer has increased greatly [69]. Polyphenolic compounds have also been shown to prevent the growth of cancer cells. In the study by Hsieh and Wu [70], the combination of epigallocatechin gallate, resveratrol, and γ-tocotrienol was used on breast cancer cells. This combination used at suboptimal doses elicited synergism in suppressing cell proliferation, modulating gene expression, and increasing antioxidant activity, as compared to each of the three phytochemicals added alone. In vitro and in vivo studies showed that resveratrol inhibits the growth of melanoma cells. When resveratrol was administered to mice, it reduced the growth of established melanoma cells and prolonged survival [71]. In a study by Nivelle et al. [72], resveratrol and various oligomeric derivatives were obtained from elicited grapevine cell suspensions. Four stilbenes (resveratrol, ε-viniferin, pallidol, and newly characterized dimer (6)) were recovered and assessed for their biological activity on the cell growth of two human skin malignant melanoma cancer cell lines (HT-144 and SKMEL-28) and a healthy human dermal fibroblast HDF line. The obtained results showed that resveratrol has the best anti-cancer properties because its efficiency against cancer cell viability is not affected by the presence of fetal bovine serum (FBS). Furthermore, resveratrol showed a tumor-specificity. Oligomers such as ε-viniferin and dimer (6) greatly reduced cancer cell viability, although this activity was considerably decreased in the presence of FBS. Anthocyanins can also have anticancerogenic activity. Cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, and an anthocyanin-rich grape extract were used to treat colon and breast cancer cell lines. They were used alone or in the presence of a clinically used drug entacapone. Entacapone in combination with anthocyanins had a greater than additive effect on the growth inhibition of the colon cancer cells. Similar results were obtained with treated breast cancer cells, where entacapone enhanced the growth inhibitory activity of the anthocyanin extract. The drug also had antiproliferative effects when used as a single treatment. It was also shown that an important mechanism for growth inhibition is oxidative stress [73]. Cyanidin-3-O-β-glucopyranoside (C3G) was shown to have anti-proliferative and pro-differentiation properties. Treatment with C3G on two different prostate cancer cells displayed reduced cell viability and increased the levels of tumor suppressor [74]. Another study on prostate cancer cell lines was conducted using grape powder extract (GPE) [75]. The GPE treatment inhibited the cell viability and growth of prostate cancer cells only at 100 µg/mL concentrations. However, at low 1.5–15 µg/mL concentrations, GPE considerably reduced the colony formation and wound healing capabilities of prostate cancer cell lines. A natural red wine extract, containing polyphenols, flavonoids, and anthocyanins, was studied on the colon cancer cell line. The red wine extract can activate molecular pathways, involving Nrf2 signaling and the modulation of structural and signaling sphingolipid mediators that cooperate in promoting differentiation and reducing the proliferation of digestive tract cancer cells [76]. In another study, the peel and seed extract were incubated with human epidermoid carcinoma A431 cell lines to evaluate antiproliferative, apoptotic effects, and the morphological apoptotic changes induced by the extracts [77]. The study demonstrated that seed and peel extracts can inhibit the growth of A431 skin cancer cells by inducing cytotoxicity, generating reactive oxygen species followed by loss of mitochondrial membrane potential, and induction of apoptosis. Another study investigated the in vitro effects and putative action mechanisms of grape seed extract (GSE) on human breast cancer cells (MCF-7). The phenolic compounds were able to induce apoptotic cell death in MCF-7 cells at suitable concentrations. At the same time, GSE induced transient but considerable enhancement of GJIC in non-communicating MCF-7 cells and an early and dose-dependent re-localization of the connexin 43 (Cx43) proteins on the plasma membrane. The gap-junction-mediated cell-cell communications (GJIC) is a basal cellular function strictly related to the carcinogenic process and should be considered a target for potential chemotherapeutic compounds [78].
One of the pharmacologically important properties of polyphenols and phenolic acids is their antimicrobial activity, i.e., antibacterial, antifungal, and antiviral activity. Their antibacterial activity has been the most researched. They can act through several mechanisms on numerous Gram-positive and Gram-negative bacteria. The mechanisms of antibacterial action of phenols include inhibition of intracellular enzymes, removal of substrate for bacterial growth, direct action on metabolic pathways such as oxidative phosphorylation or electron transfer, and prevention of metalloprotein synthesis through metal ion complexation. Inhibition of nucleic acid synthesis in certain bacteria is based on the inhibition of enzymes such as DNA gyrase and DNA topoisomerase. Due to their structure, phenols can interact with proteins, lipids, and certain enzymes of bacterial cell membranes, and thus cause changes in membrane functionality in the form of changes in fluidity and permeability, allowing the loss of protons, ions, and macromolecules, but also allowing other molecules to enter the cell, such as antibiotics. Oxidative phosphorylation is an important metabolic process for obtaining energy in the form of ATP molecules. Inhibition of only one of the enzymes of this process, such as NADH-cytochrome c reductase, inhibits the whole process, which inhibits the growth of bacteria. Some phenols can inhibit the human immunodeficiency virus (HIV) and in particular the pandemic strain of HIV-1 by preventing the virus from entering the host cell or preventing its replication by inhibiting key enzymes, such as HIV-1 reverse transcriptase and other DNA polymerases. Numerous phenolic compounds can also inhibit the activity of other viruses: herpes simplex virus, adenoviruses, respiratory syncytial virus, poliovirus, rabies virus, rotavirus, and Sindbis virus. Regarding those viruses, the polyphenols inhibit various DNA or RNA polymerases [79][80][81][82][83][84][85][86].