1000/1000
Hot
Most Recent

Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease.
Lung cancer is the most common cancer, contributing 11.6% of the total case number, and is responsible for 18.4% of cancer-related deaths around the world [1][2]. Despite the social effort to identify high-risk populations by lung cancer screening and the development of prediction tools, as well as prevention campaigns, the incidence of lung cancer is expected to increase by 71.4% by 2040 worldwide [3]. It has been estimated that approximately 1 in 15 people will develop lung cancer throughout their lives [4]. In addition, according to sex, it is the most prevalent tumor in men and the third most prevalent tumor in women. Although lung cancer causes more deaths every year than all breast, prostate and colorectal tumors combined according to the American Cancer Society, the good news is that the decline in lung cancer mortality has been accelerating by 2% in the last decade for both men and women [4].
Tobacco smoking is the leading risk factor associated with this disease, with 80% of cases attributed to it in Western countries. Nevertheless, this pattern shows up to 20-fold variation in lung cancer rates from one country to another, especially according to the level of development and socioeconomic status of the region, where the rates of pollution also play an important role [5]. Thus, it is more common in developed countries, especially in the USA and Europe, and less frequent in less developed countries, such as Africa and South America [6]. Moreover, exposure to secondhand smoke, radon gas, asbestos, infections and genetic susceptibility are other common risk factors for lung cancer [2]. Individual susceptibility, which derives from different human polymorphisms present in the human population, affects the balance between metabolic activation, detoxification, and reparation of DNA adducts differently [7][8].
This heterogeneity among patients has led to the establishment of different subgroups according to morphological, immunohistochemical and genetic characteristics. Thus, lung cancer is classified into two main groups, each ranging from stage I to IV depending on tumor progression: small cell lung cancer (SCLC) (15% of patients) and non-small cell lung cancer (NSCLC) (80% of patients). The latter is, in turn, subdivided into three subtypes: large cell carcinoma (LCC, 10% of all cases), squamous cell carcinoma (SCC, 25%), and lung adenocarcinoma (ADC, 40%). Between the main histological subtypes of NSCLC, studies have shown that SCC tends to arise centrally within the main or lobar bronchus, showing slow growth, and its prevalence is highly associated with tobacco smoking, which increases the mutational burden by 10 times [9]. In these tumors, the proposed actionable genes with clinical efficacy are limited. On the other hand, ADC appears more peripheral, mainly affecting distal bronchioli and alveoli, with glandular and mucin differentiation. ADC is the most common subtype among never smokers and women [10], and although it generally has a worse prognosis, it is frequently associated with druggable driver mutations such as epidermal growth factor receptor (EGFR) mutation and echinoderm microtubule associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion protein, among others, for which there are targeted therapies with good clinical results [2]. In turn, SCLC is frequently located centrally in the lung and presents a poor prognosis. Consequently, the implementation of genetic characteristics for the histological classification of cancer opens the possibility of developing novel targeted therapies and therefore optimizing precision medicine [11].
The treatment of lung cancer depends on several factors, such as the condition of the patients, histological features, and tumor TNM staging. Classical treatment options include surgical resection, platinum-based chemotherapy and radiation therapy, in monotherapy or in combination, as well as sequential therapeutic strategies, among others. Surgery is the standard option for early-stage lung cancer, when the cancer is confined to the lung and therefore considered to be curable [12]. These stages involve potentially resectable lesions, macroscopically or microscopically, offering more guarantees of control or cure of the disease [13]. In intermediate stages, surgery is only for diagnostic purposes because at this point, the disease becomes difficult to control [14]. Despite surgery, the recurrence rate remains high in the early stages of NSCLC, and 30–55% of patients with curative resection develop recurrences that occur mainly at a distance [15]. In very advanced cases, that is, stages associated with distant metastases, surgery only has indication with palliative or diagnostic effects, and systemic anticancer treatments are applied in an attempt to slow tumor growth and improve overall survival (OS) and the quality of life of the patient and minimize symptoms [16]. Regarding systemic treatments, chemotherapy (CT) is the first option in patients with SCLC. However, in patients with NSCLC, it is used as a complementary strategy to surgery, where it can be given before (neoadjuvant) or after (adjuvant) surgery for curative or palliative purposes. In early-stage NSCLC, radiotherapy (RT) can be used instead of surgery with curative effects. After surgery in stage II and IIIA patients, adjuvant CT has proven to prevent recurrence [17], while unresectable stage III lung tumors are recommended to be treated with CT-RT. In the case of metastatic cancer, treatment with CT has been established, but RT is also used for palliative care of symptoms [18][19]. In the case of SCLC, treatment consists of a combination of platinum-based CT with etoposide. In addition, RT will be used in cases of localized disease and for the prevention and treatment of brain metastases.
However, despite the results obtained with CT and RT in lung cancer, they have many undesirable side effects, and the mortality and morbidity data are still very high, with 5-year OS rates of approximately 10-15%, making it the most lethal neoplasm [1]. Approximately 25% of lung cancer cases harbor genetic abnormalities amenable to treatment with targeted therapies, improving the survival time of patients. This has allowed targeted therapies to appear in clinical oncology practice, improving the survival rates and quality of life of patients. These therapies are treatments that selectively target cancer-specific genes, proteins, or the tissue environment that contribute to cancer growth and survival, blocking them and minimizing damage to healthy cells [20]. Many of these targeted therapies focus on various tyrosine kinase receptors (RTKs) that are involved in cell growth and survival, whose alterations amplify the signals that lead to tumorigenesis. Thus, RTK inhibitors (TKIs) are used to interrupt these signaling pathways, but their use tends to produce acquired resistance [21]. Successful biomarkers with therapeutic purposes in patients with ADC have been mainly EGFR mutations, ALK- and RET- (rearranged during transfection) gene rearrangements, ROS1 (receptor tyrosine kinase ROS proto-oncogene 1) fusions; V600E-specific mutation of BRAF (v-Raf murine sarcoma viral oncogene homolog B) gene; MET (hepatocyte growth factor receptor) factor amplification, KRAS and HER2 (human epidermal growth factor receptor 2) gene mutations, among others [16][21][22][23]. In contrast, patients with SCC show few alterations for therapeutic purposes. FGFR1 (fibroblast growth factor receptor 1) amplification or PI3K (phosphoinositol 3-kinase) mutations are challenging targets but with no effective inhibitors yet [24]. However, the limited understanding of SCLC molecular biology has led to completely nonexistent targeted treatment today. In addition, it is important to highlight that targeted therapies in patients with lung cancer may fail or have little or no efficacy when treatments are carried out in populations that are not molecularly selected.
The greatest challenge for lung cancer clinical management has become markedly evident with the use of immunotherapy (IT). There is increasing evidence of the role of the host immune system in immunosurveillance and tumor rejection in which every known innate and adaptive immune effector mechanism participates. Moreover, may be transitory or long-lasting a crucial role so that local immunosuppression in a tumor context caused by chronic inflammation has protumor effects, whereas enhancing T-cell function and dendritic cell maturation derived from acute inflammation has the opposite (antitumor) effect [25]. Thus, the immune system has been recognized as another important cancer hallmark [26]. This has led to the development of therapeutic strategies focused in this direction, such as molecules for reactivating the host immune response as cancer vaccines or checkpoint inhibitors to emphasize intrinsic antitumor immunity [27]. However, a high percentage of patients with lung cancer do not respond to these treatments, or their responses may be transitory or long-lasting. Here, we review the relevance of immunotherapy in lung cancer, with a focus on the underlying resistance mechanisms of the disease.
Immuno-oncology has emerged as a promising field that seeks to reinforce the host’s own immune system to avoid immune evasion of the tumor by recognition of tumor-specific antigens (neoantigens) and tumor-associated antigens (TAAs) that trigger an immune reaction that causes tumor remission [28]. Therefore, attacking tumor cells directly is no longer the target of the therapy, but the strategy is redirected towards the immune system. This makes it work for any tumor histology or driver mutation, and the side effects are different from other therapies [29]. Accordingly, cancer immunotherapy has been developed based on several approaches, ranging from stimulating effector mechanisms to counteracting inhibitory and suppressive mechanisms. Vaccines are among the strategies to activate immune effector cells, while additional stimulation strategies include cytokines, adoptive cell therapy and oncolytic viruses. On the other hand, the use of antibodies against immune checkpoint molecules stands out as one of the strategies to neutralize immunosuppressive mechanisms [30] (Figure 1).
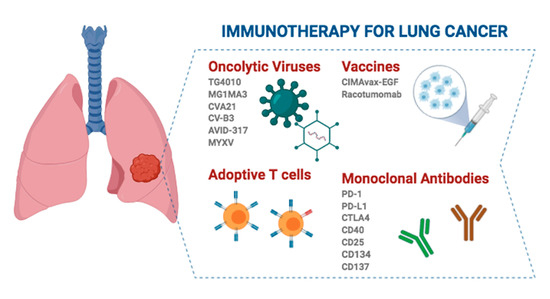
Figure 1. Different strategies and immunotherapeutic agents with clinical application in lung cancer. Created with Biorander.com.
Thus, since the activation of the immune checkpoint pathways is a very frequent tumor evasion mechanism, the use of inhibitors against immune checkpoints (ICIs) arises as IT, stimulating antitumor responses towards tumor-specific antigens within the TME, which is composed of stromal cells, immune cells, and extracellular matrix, all of which are closely related to tumor cells. The rate of tumor-infiltrating immune cells in the TME can be classified as immunodesert (noninfiltrated or also called ‘immunologically cold’), immunoexcluded (peripheral immune infiltration around tumor cells) and immunoinflammed (infiltrated or also called ‘immunologically hot’). The latter expresses immune checkpoint molecules and is correlated with a more favorable response to ICI therapy [31].
Because IT has promising results in terms of survival and quality of life, more treatments of this type for lung cancer are trying to be developed, since it is frequently diagnosed in advanced stages, when other classical therapies such as surgery, chemotherapy, and radiotherapy are minimally effective. Nonetheless, IT can be combined with any of the therapeutic modalities already described. While the synergistic effects of combining these drugs are being evaluated, they do not produce additional toxicities or death [29].
Nonspecific immunotherapies, also known as immunomodulatory therapies, are not directed against a specific antigen but rather aim to bolster the antitumor immune response. They usually involve both the innate and adaptive responses of the immune system, and their mechanisms of action can be direct antitumor effects, reversing immunosuppression, activating innate immunity, and activating antigen-nonspecific T-cells [32]. These treatments include cytokines (interleukins and interferons) as the most frequently used compounds, although there are others, such as immune-stimulatory agents (CpG oligonucleotides or Bacille Calmette-Guérin, BCG), antibodies and enzyme inhibitors [32]. The two Food and Drug Administration (FDA)-approved cytokines for the treatment of severe malignancies are IL-2 and IFN-α; however, neither of them has an indication in lung cancer.
Tumors create an immunosuppressed microenvironment that allows them to escape from the immune system, but in turn, this makes tumors more sensitive to viral infections. Oncolytic virotherapy, which uses attenuated and genetically modified viruses capable of selectively infecting tumor cells, is based on this premise, taking advantage of the deregulated pathways to produce cell lysis. The second mechanism of action of OVs is the induction of antitumor immunity, which takes place thanks to the antigens released during oncolysis. The third is the ability to produce acute vascular-disrupting effects that lead to tumor reduction [33]. Thus, in OV therapy, the virus is the active agent itself and not a carrier, as occurs in gene therapy [34]. The advantages of oncoviral immunotherapy are its specificity targeting tumor cells, its independence of specific receptor expression patterns and therefore of the associated resistances, and its ability to enhance the antitumor immune response or induce a novel nonself antigen response [35].
Some of the viruses that are being considered for this type of immunotherapy are from the herpes simplex family, such as fowlpox virus, Newcastle disease virus, reovirus and measles virus (MV), but some adenoviruses, picornaviruses (including coxsackie), reovirus, maraba, vaccinia virus, retroviruses and mumps are also considered [35][36][37]. In 2015, the FDA approved talimogene laherparepvec (T-VEC or Imlygic), a second-generation oncolytic herpes simplex virus type 1 (HSV-1) armed with Granulocyte Macrophage colony-stimulating factor (GM-CSF) [34], for the treatment of metastatic melanoma, which was the first approved oncolytic viral immunotherapy, although there are several clinical trials of oncolytic viruses that cover almost all solid tumors, including lung cancer.
Today, there are some OVs garnering intense interest for use in lung cancer clinical practice. This is the case for TG4010, which is a modified vaccinia virus Ankara designed to express MUC-1 and IL-2 that is being evaluated in a phase III clinical trial for advanced NSCLC. In addition, it has been reported that TG4010 in combination with CT improves the progression-free survival of patients and increases durable responses and long-term survival. Furthermore, there is evidence pointing to a synergistic effect when combined with anti-PD-1/PD-L1 (programmed cell death protein 1 and programmed death ligand 1, respectively) ICIs [37][38][39]. On the other hand, the oncolytic vaccine MAGE-A3 is also being evaluated in two phase I/II clinical trials. One of them uses the MG1 Maraba/MAGE-A3 (MG1MA3) virus alone or in combination with the adenovirus/MAGE-A3 (AdMA3) virus in patients with incurable advanced MAGE-A3-expressing solid tumors (NCT02285816; https://clinicaltrials.gov). Another clinical trial evaluated the combination of MG1MA3 + AdMA3 + pembrolizumab in previously treated patients with metastatic NSCLC (NCT02879760; https://clinicaltrials.gov). Inoculation of the antigen-expressing adenovirus that is also encoded within the OV results in T-cell-mediated tumor clearing and protection from relapses in vivo, avoiding an antiviral response [40]. Additionally, coxsackievirus A21 (CVA21, CAVATAK), which targets ICAM-1 naturally and in combination with pembrolizumab, is well tolerated and appears to increase the number of PD-L1+ tumor cells in a phase Ib clinical trial [41]. Another coxsackievirus, type B3 (CV-B3), is a nonenveloped, human-pathogenic enterovirus that produces a significant reduction in cell survival in KRAS-mutant NSCLC cell lines [42]. Adenovirus AVID-317 has shown effectiveness in 70% of tested human NSCLC-derived cell lines and an increase in the median survival in murine models [43]. Similarly, infection of NSCLC cells by MV induces tumor apoptosis in vitro and reduces tumor size in mice [44]. In SCLC, there are also OV studies, specifically with a modified oncolytic myxoma virus (MYXV), in murine models. This strategy produces tumor-specific cytotoxicity, necrosis mediated by immune cell infiltration and increased survival [45]. Despite these promising results, further studies are needed to optimize the delivery of vectors, as well as to obtain OVs with precise coordination with the immune system to effectively eradicate the tumor.
In adoptive cell transfer, tumor-reactive lymphocytes from the patient are collected, cultured ex vivo and reinfused, often along with growth factors, into the patient as therapy with the goal of recognizing, targeting, and destroying tumor cells. The cytotoxic T lymphocytes (CTLs) that can be used for this are tumor-infiltrating lymphocytes (TILs), T-cell receptor (TCR)-modified T-cells and chimeric antigen receptor (CAR)-modified T-cells [46][47][48]. In the latter, the cells are genetically engineered to target tumor-specific surface antigens. The advantages of this strategy [48] are that it is highly specific towards tumor cells; has a robust clonal expansion ability; presents tropism towards the antigen, so it can migrate towards metastasis; generates memory, maintaining long-term effectiveness; and under the right therapeutic conditions, adoptive cell therapy can eradicate tumors.
The FDA approved the use of CAR-T-cells, making them the first genetically engineered modified cell therapy with this approval. However, despite the good results in hematological tumors, their application in solid tumors has been largely limited. This is believed to be due to difficulties in finding specific targetable antigens, T-cell homing, infiltration and survival in the TME [47]. Nevertheless, strategies are already being developed to overcome these obstacles, such as the split, universal, and programmable (SUPRA) CAR system, which uses universal receptors that allow target multiplexing and implements multiple advanced logic and control features [49]. The application of this technology has not been very extensive in the treatment of patients with NSCLC, caused among other reasons by the fact that choosing an appropriate target is a challenge. Notwithstanding, there are currently 5 ongoing clinical trials (NCT04153799, NCT03525782, NCT03029273, NCT04025216 and NCT02706392; https://clinicaltrials.gov) trying to validate these new treatment options. However, despite being a powerful tool in nonresponsive patients and immunologically cold tumors, it is a high-cost individualized medication, which is currently limiting its use in the general population.
The idea of using cancer vaccines emerged in the late 19th century from the observation that some tumors remitted spontaneously after the patient suffered an infectious disease. Thus, vaccines are designed as an active specific immunotherapy that stimulates the immune response by presenting a pathogen or TAAs and producing an adaptive antitumor response. This boosts tumor antibodies and T-cells in vivo in a similar way to passive immunotherapy (i.e., tumor-specific antibodies or T-cells) [50]. In addition, they can be used for both treatment and prevention and may or may not be combined with other therapies.
Several types of tumor vaccines have been investigated, such as (i) allogeneic vaccines in which antigens come from non-self-cancer cells to stimulate the cytotoxic immune response; (ii) antigens or protein-based vaccines; (iii) autologous dendritic cell vaccines in which self-dendritic cells (DCs) are activated with tumor antigens; iv) DNA vaccines in which an expression plasmid harbors the target antigen; and (v) vector-based vaccines in which antigens are administered through special viruses, bacteria, yeast cells or other structures. The most widely used vaccines are peptide-based vaccines with immunogenic epitopes, usually from tumor-specific or tumor-associated antigens. This strategy typically uses synthetic peptides, DNA or RNA to encode the neoantigen; however, the fact that these neoantigens are not universal for a type of tumor or a group of patients limits their widespread use and leads to the development of tailored and even polyneoantigen vaccines [47].
Despite efforts, only one vaccine has been approved by the FDA, namely, the therapeutic DC-based cancer vaccine Sipuleucel-T (Provenge™), for the treatment of metastatic castration-resistant prostate cancer [51]. However, in some Latin American countries, the CIMAvax- Epidermal Growth Factor (EGF) and Racotumomab vaccines have been approved for advanced NSCLC [46]. Despite the fact that the use of CIMAvax-EGF in NSCLC seems to have a tendency towards clinical benefit and to be immunogenic, the phase III randomized trials carried out have not been able to demonstrate sufficient efficacy and survival impact to include it in the protocols of NSCLC treatment [52]. In the case of racotumomab, approximately 20–25% of patients who received this therapeutic vaccine in a phase II/III study did appear to have great clinical benefit with longer progression-free survival (PFS) and OS [53][54]. However, these strategies are not supported as clinical approaches to patients with lung cancer.
Monoclonal antibodies recognize a single epitope region by a pair of variable domains (fragment of antigen binding, Fab) [55]. Antibodies are involved in cell lysis, which is caused by antibody-dependent cell-mediated cytotoxicity (ADCC) carried out by immune effector cells such as natural killer cells, neutrophils, mononuclear phagocytes and DCs. In addition, antibodies are also key in complement-dependent cytotoxicity (CDC) and the induction of adaptive immune responses, which intervene in the long-term benefit of these treatments through the presentation of tumor-derived peptides on MHC class II molecules (activating CD4+ T-cells) and on MHC class I molecules (activating CD8+ cytotoxic T-cells).
mAbs are produced as chimeric, humanized or human antibodies by recombinant DNA hybridomas. This is done to avoid immunogenicity of the original murine antibodies, which decrease the efficacy due to the human anti-mouse antibody response [51]. Depending on the type of antibody, a different suffix will be added to the name of the treatment. Thus, murine mAbs will use the suffix -omab, chimeric mAbs will end in -ximab, humanized mAbs in -zumab and human mAbs in -umab [56]. In turn, mAbs can be self-acting nacked (the most frequent in nonleukemic cancers), having a therapeutic function by targeting growth factor receptors, or conjugated, when they are combined with chemotherapeutic drugs or radioactive isotopes. Nacked mAbs work either by labeling tumor cells, by targeting immune system checkpoints or by blocking tumor antigens involved in cell growth and spread. Meanwhile, the conjugated mAbs act as a specific delivery system. On the other hand, there are bispecific mAbs capable of recognizing two different epitopes, normally one in tumor cells and the other in immune effector cells, which bring them closer together. To overcome its clinical limitations due to their short half-life and toxicity, a modification known as BiTE (bispecific T-cell engager) molecules has been developed, as well as engineered protein scaffolds with antitumor activity [55].
Different mechanisms of action have been attributed to mAbs with antitumor effects (Table 1 and Table 2). First, they can function as targeted therapy when designed against a specific tumor molecular target. In this case, when the type and location of the tumor do not define the treatment but depend only on the molecular target, it is called tumor-agnostic treatment. The first drug approved by the FDA with this indication was pembrolizumab, an anti-PD-1 antibody used to treat patients with unresectable metastatic solid tumors with microsatellite instability-high (MSI-H) or DNA mismatch repair deficiency (dMMR). Second, mAbs can activate the patient’s immune system to destroy tumor cells. This usually consists of unblocking certain pathways that are altered as a tumor evasion mechanism by targeting immunoregulatory coreceptors, reversing tumor immunosuppression and modulating the constant fragment (Fc) domain of mAbs. Ipilimumab, a cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-specific mAb that enhances the effector functions of T-cells while inhibiting Regulatory T cells (Tregs), stands out among these immune cell-targeting therapeutic strategies in NSCLC. However, there are also other approaches for different tumors, including antibodies targeting CD40, CD25, CD134, CD137, etc. (Table 1 and Table 2). Fc domain modifications are accomplished by mutations resulting in improved ADCC, such as in ocrelizumab (anti-CD20), or by modification of the oligosaccharide content (defucosylation) [55]. Finally, blocking ligand binding to growth factor receptors and/or their signaling pathways, as well as targeting the tumor microenvironment, through the inhibition of angiogenesis, cytokines, and growth factors, are other therapeutic strategies with increasing chances of success [55]. Examples of these latter types of antibodies are nintedanib, a triple angiokinase inhibitor used as a second-line therapy in combination with taxotere, and bevacizumab (a humanized VEGF-specific mAb), which was the first approved agent against tumor angiogenesis [57].
Table 1. NSCLC tumor-associated antigens targeted by monoclonal antibodies that are currently being tested in clinical trials.
| Antigen Target | Monoclonal Antibody Name | Clinical Trial ID (https://clinicaltrials.gov) |
|---|---|---|
| B7-H3 | Enoblituzumab (MGA271) | NCT02475213 |
| BTLA | TAB004 | NCT04137900 |
| CD137 | BMS-663513 | NCT00461110 |
| CD40 | APX005M | NCT02482168 |
| SEA-CD40 | NCT02376699 | |
| CD44 v6 | Bivatuzumab | NCT02204059 |
| CD73 | CPI-006 | NCT03454451 |
| Oleclumab | NCT04262388 | |
| CEA | Yttrium Y 90 anti-CEA monoclonal antibody cT84.66 | NCT00738452 |
| Yttrium Y 90 anti-CEA monoclonal antibody MN-14 (90Y-hMN-14), indium In 111 anti-CEA monoclonal antibody MN-14 | NCT00006458 | |
| CEACAM1 | CM-24 | NCT02346955 |
| c-MET | Sym015 | NCT02648724 |
| CSF1R | Cabiralizumab | NCT03502330 |
| CTLA-4 | Ipilimumab * | NCT03001882, NCT02350764 |
| ONC-392 | NCT04140526 | |
| REGN4659 | NCT03580694 | |
| Tremelimumab | NCT02542293, NCT02000947 | |
| DLL3 | Rovalpituzumab tesirine | NCT03000257 |
| DLL4 | Demcizumab | NCT01189968 |
| EpCAM | Tucotuzumab celmoleukin | NCT00016237 |
| ErbB1/EGFR | Cetuximab | NCT00986674 |
| Futuximab/modotuximab (Sym004) | NCT02924233 | |
| Necitumumab * | NCT02496663 | |
| Nimotuzumab | NCT01393080 | |
| Matuzumab | NCT00111839 | |
| Panitumumab (ABX-EGF) | NCT00034346 | |
| Pertuzumab | NCT03845270 | |
| SCT200 | NCT03808701 | |
| ErbB2/HER2 | Trastuzumab (Herceptin) | NCT04285671, NCT03505710, NCT03845270 |
| ErbB3/HER3 | Seribantumab (MM-121) | NCT02387216, NCT00994123 |
| GDF15 | NGM120 | NCT04068896 |
| GM2 Ganglioside | BIW-8962 | NCT01898156 |
| HGF | Ficlatuzumab (AV-299) | NCT01039948 |
| ICOS | GSK3359609 | NCT03693612 |
| KY1044 | NCT03829501 | |
| Vopratelimab | NCT03989362 | |
| IGF-1, IGF-2 | Xentuzumab | NCT02191891 |
| IGF-1R | Cixutumumab | NCT00778167, NCT00986674 |
| Dalotuzumab | NCT00951444 | |
| Figitumumab (CP-751,871) | NCT00560573 | |
| Ganitumab (AMG 479) | NCT00807612 | |
| IL1RAP | Nidanilimab (CAN 04) | NCT03267316 |
| LAG-3 | TSR-033 | NCT02817633 |
| LIF | MSC-1 | NCT03490669 |
| Mesothelin | Amatuximab | NCT00325494 |
| Anetumab Ravtansine | NCT03455556 | |
| LMB-100 | NCT04027946 | |
| CD134 | INCAGN01949 | NCT02923349 |
| PD-1 | BCD-100 | NCT03288870 |
| Budigalimab (ABBV-181) | NCT03000257 | |
| Camrelizumab (SHR-1210) | NCT03527251 | |
| Cemiplimab | NCT03580694 | |
| Dostarlimab | NCT02715284 | |
| Nivolumab * | NCT04043195, NCT04023617 | |
| Pembrolizumab * | NCT04393883, NCT03053856 | |
| Retifanlimab (MGA012) | NCT02475213 | |
| Sasanlimab (PF-06801591) | NCT04181788 | |
| SCT-I10A | NCT04171284 | |
| Serplulimab (HLX10) | NCT04033354 | |
| Sintilimab | NCT03812549 | |
| Spartalizumab | NCT04323436, NCT04000529 | |
| Tislelizumab | NCT03358875 | |
| Toripalimab | NCT04158440, NCT04304248 | |
| Zimberelimab (AB122) | NCT04262856, NCT03629756 | |
| PDGF-R α | Olaratumab | NCT00918203 |
| PD-L1 | Adebrelimab (SHR-1316) | NCT04316364 |
| Atezolizumab * | NCT03977467, NCT03645330 | |
| Avelumab | NCT03158883 | |
| Cosibelimab | NCT03212404 | |
| Durvalumab * | NCT02000947, NCT03694236 | |
| Sugemalimab (CS1001) | NCT03789604 | |
| TQB2450 | NCT03910127 | |
| Phosphatidylserine (Ptd-L-Ser or PS) | Bavituximab | NCT01160601, NCT01138163 |
| PSMA | 177Lu-J591 | NCT00967577 |
| RAAG12 | RAV12 | NCT00101972 |
| sCLU | AB-16B5 | NCT04364620 |
| SEMA4D | Pepinemab | NCT03268057 |
| TF | MORAb-066 | NCT01761240 |
| TGFB | Fresolimumab | NCT02581787 |
| TIM-3 | Cobolimab (TSR-022) | NCT02817633 |
| INCAGN02390 | NCT00994123 | |
| MBG453 | NCT02608268 | |
| TRAIL-R1 | TRM-1 (HGS-ETR1) | NCT00092924 |
| TRAIL-R2 | Conatumumab (AMG 655) | NCT00534027 |
| VEGF | Bevacizumab * | NCT03836066, NCT03779191 |
| GB222 | NCT04175158 | |
| LY01008 | NCT03533127 | |
| QL1101 | NCT03195569 | |
| VEGFR2 | Alacizumab pegol (CDP791) | NCT00152477 |
| Ramucirumab (IMC-1121B) * | NCT01160744 | |
| α5β1 integrin | Volociximab | NCT00666692 |
* Monoclonal antibodies approved by the FDA for NSCLC.
Table 2. SCLC tumor-associated antigens targeted by monoclonal antibodies that are currently being tested in clinical trials.
| Antigen Target | Monoclonal Antibody Name | Clinical Trial ID (https://clinicaltrials.gov) |
|---|---|---|
| BEC-2 | Mitumomab | NCT00037713 |
| CD56 | Lorvotuzumab mertansine | NCT00346385 |
| CEA | Yttrium Y 90 anti-CEA monoclonal antibody MN-14 (90Y-hMN-14), indium In 111 anti-CEA monoclonal antibody MN-14 | NCT00006347 |
| CTLA-4 | Ipilimumab | NCT03575793 |
| Tremelimumab | NCT02701400 | |
| DLL3 | 89Zr-DFO-SC16.56 | NCT04199741 |
| Rovalpituzumab Tesirine | NCT03000257 | |
| EpCAM | Tucotuzumab celmoleukin | NCT00016237 |
| ErbB1/EGFR | Cetuximab | NCT00104910 |
| ErbB2/HER2 | Trastuzumab (Herceptin) | NCT00028535 |
| GD2 ganglioside | Dinutuximab | NCT03098030 |
| MOAB 3F8 | NCT00003022 | |
| GD3 ganglioside | Mitumomab | NCT00006352 |
| GM2 Ganglioside | BIW-8962 | NCT01898156 |
| IGF-1R | Cixutumumab | NCT00887159 |
| Dalotuzumab | NCT00869752 | |
| Lewis-Y | Hu3S193 | NCT00084799 |
| PD-1 | Budigalimab (ABBV-181) | NCT03000257 |
| Camrelizumab (SHR1210) | NCT03755115, NCT03417895 | |
| Nivolumab * | NCT03382561 | |
| Pembrolizumab | NCT03319940 | |
| Serplulimab (HLX10) | NCT04063163 | |
| PD-L1 | Atezolizumab * | NCT03262454 |
| Durvalumab * | NCT02701400 | |
| TQB2450 | NCT04234607 | |
| ZKAB001 | NCT04346914 | |
| TAA | Bevacizumab | NCT00079040 |
| TIM-3 | INCAGN02390 | NCT03652077 |
| VEGF | SC-002 | NCT02500914 |
* Monoclonal antibodies approved by the FDA for SCLC.
Currently, neutralizing monoclonal antibodies targeting immune checkpoints such as CTLA-4 and PD-1/PD-L1 have shown significant efficacy against various types of cancer, including NSCLC. Anti-CTLA-4 was the first immune checkpoint antagonist available for NSCLC; however, it has shown higher toxicity and less effectiveness than anti-PD-1/PD-L1 treatments, the latter being the most successful to date. Currently, four of these ICIs have been approved for NSCLC: nivolumab and pembrolizumab (both anti-PD-1) and atezolizumab and durvalumab (both anti-PD-L1) [28] (Table 1).
In the case of SCLC, ICIs appear to be a promising therapy, and numerous clinical trials are underway to test them (Table 2). Thanks to the results of the CheckMate-032 study, the FDA approved the use of nivolumab as a third-line therapy for metastatic SCLC in 2018. The following year, atezolizumab with carboplatin and etoposide as first-line therapy and durvalumab in combination with CT in 2020 were approved for extensive-stage SCLC by the FDA [58].
Despite the great advances achieved through the use of ICIs, not all patients with lung cancer respond to this treatment due to primary resistance and the development of secondary resistance. As a result, long-lasting clinical remission only represents a small percentage of outcomes. For this reason, great effort is being made to find predictive and monitoring biomarkers [28].