1000/1000
Hot
Most Recent

Endocrine disruptors (EDs) are contaminants that may mimic or interfere with the body’s hormones, hampering the normal functions of the endocrine system in humans and animals. These substances, either natural or man-made, are involved in development, breeding, and immunity, causing a wide range of diseases and disorders. The traditional detection methods such as enzyme linked immunosorbent assay (ELISA) and chromatography are still the golden techniques for EDs detection due to their high sensitivity, robustness, and accuracy. Nevertheless, they have the disadvantage of being expensive and time-consuming, requiring bulky equipment or skilled personnel. On the other hand, early stage detection of EDs on-the-field requires portable devices fulfilling the Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment free, Deliverable to end users (ASSURED) norms. Electrochemical impedance spectroscopy (EIS)-based sensors can be easily implemented in fully automated, sample-to-answer devices by integrating electrodes in microfluidic chips.
Endocrine disruptors (EDs) are environmental contaminants that disrupt the normal functioning of the endocrine system in mollusk, crustacea, fish, reptiles, birds, and mammals. For humans, these compounds may cause cancerous tumors [1][2][3] and infertility [4]. Natural EDs originate in living organisms and can be either hormones (testosterone, estrogen, or progesterone) or mycotoxins such as zearalenone. Synthetic EDs can be found in plastic additives, industrial reagents, and waste. Some of the most common synthetic EDs are precursors in the production of rubber, pesticides and plastic additives such as atrazine, alkylphenols, bisphenol A (BPA) [5], parabens, perfluoroalkyl acids [6], phthalates and polychlorinated biphenyls (PCBs) [7]. A list of relevant EDs is given in Table 1.
A variety of analytical methods have been used for the detection of EDs, including liquid chromatography coupled with mass spectrometry (LC-MS) [8], gas chromatography coupled with mass spectrometry (GS-MS) [9], high-performance liquid chromatography (HPLC) coupled with fluorescence detection [10] or with mass spectrometry [11][12]. These methods usually require laborious and time-consuming steps for sample pre-concentration, and high amounts of reagents. By comparison, electrochemical sensors and biosensors offer advantages such as low cost, portability, and do not require complex pretreatment steps. Moreover, biosensors can be used for selective, fast, and direct detection of analytes in real samples.
Table 1. List of relevant EDs compounds.
| Analyte | IUPAC Name | Chemical Structure | Molecular Weight (g/mol) | Source | Ref. |
|---|---|---|---|---|---|
| 17β-estradiol (E2) |
(8R,9S,13S,14S,17S)-13-Methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol | 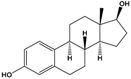 |
272.388 | endogenous hormone, medication | [13] |
| Acetamiprid (AAP) |
N-[(6-chloro-3-pyridyl)methyl]-N′-cyano-N-methyl-acetamidine | 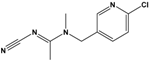 |
222.678 | insecticide | [14] |
| Atrazine (ATZ) |
6-chloro-N2-ethyl-N4-(propan-2-yl)-1,3,5-triazine-2,4-diamine | 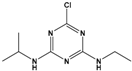 |
215.69 | herbicide for grassy weeds in crops | [15] |
| Pentabromodiphenyl ether (BDE-47) |
2,2′,4,4′-Tetrabromodiphenyl ether | 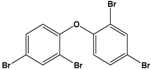 |
485.79 | flame retardant | [16] |
| Bisphenol A (BPA) |
4,4′-(propane-2,2-diyl)diphenol | 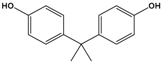 |
228.291 | precursor to polycarbonates, plastic and epoxy resins | [17] |
| Carbendazim (CBZ) |
methyl 1H-benzimidazol-2-ylcarbamate | 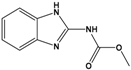 |
191.187 | fungicide | [18] |
| Cortisol | 11β,17α,21-Trihydroxypregn-4-ene-3,20-dione | 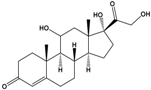 |
362.46 | endogenous hormone, medication | [19] |
| Dibutyl phthalate (DBP) |
Dibutyl benzene-1,2-dicarboxylate | 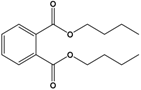 |
278.348 | plasticizer | [20] |
| Dichloro-diphenyl-trichloroethane (DDT) |
1-chloro-4-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene | 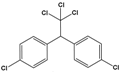 |
354.48 | pesticide | [21] |
| Di(2-ethylhexyl) phthalate (DEHP) |
Bis(2-ethylhexyl) benzene-1,2-dicarboxylate | 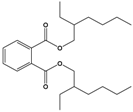 |
390.564 | plasticizer | [22] |
| Microcystin-LR (MC-LR) |
(5R,8S,11R,12S,15S,18S,19S,22R)-15-[3-(diaminomethylideneamino)propyl]-18-[(1E,3E,5S,6S)-6-Methoxy-3,5-dimethyl-7-phenylhepta-1,3-dienyl]-1,5,12,19-tetramethyl-2-methylidene-8-(2-methylpropyl)-3,6,9,13,16,20,25-heptaoxo-1,4,7,10,14,17,21-heptazacyclopentacosane-11,22-dicarboxylic acid | 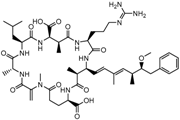 |
995.189 | cyanobacteria toxin | [23] |
| Norfluoxetine (NorFLX) |
(S)-3-Phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine | 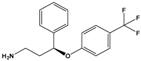 |
295.305 | antidepressant | [24] |
| 3,3’,4,4’-tetrachlorobiphenyl (PCB-77) |
3,3′,4,4′-tetrachloro-1,1′-biphenyl | 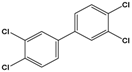 |
291.99 | flame retardants, plasticizers, dielectric and heat transfer fluids | [25] |
| Testosterone | (8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | 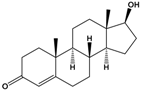 |
288.431 | endogenous hormone, anabolic steroid | [26] |
| Tributyltin hydride | tributylstannane | 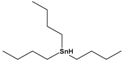 |
291.06 | precursor in organic synthesis | [27] |
| Zearalenone (ZEN) |
(3S,11E)-14,16-Dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione | 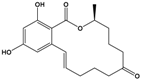 |
318.369 | mycotoxin | [28] |
Electrochemical impedance spectroscopy (EIS) is a sensitive technique which can be used to monitor biomolecular events occurring at the electrode surface. These events include affinity interactions involving peptides, receptors, nucleic acids, whole cells, and antibodies.
Immunoassays are based on the specific interaction between an antigen and the corresponding antibody (Ab), which can be transduced into a measurable physical signal [29]. Immunosensors can be prepared using monoclonal, polyclonal or recombinant Abs. Immunosensors have been used for the detection of EDs, such as DES, estradiol, phthalates and bisphenol A [30].
Aptamers are oligonucleotides that bind to a specific target. Because of their in vitro selection and production, the relatively new technology of aptamers has emerged as an alternative to antibodies, as they are obtained through chemical synthesis, with high reproducibility, and their production is not dependent on living organisms. They can be easily regenerated, have a much longer shelf life, and can be stored at ambient temperature.
Human-estrogen receptor alpha (ER-α) is a protein that belongs to the nuclear receptor group and can bind xenoestrogens such as 17β-estradiol. Due to its specificity and ability to be engineered, ER-α was used as bio-recognition element for the development of ED detection methods [31]. Estrogen receptor-based biosensors are not often encountered.
Other biorecognition elements, such as enzymes, were used to develop the ED biosensors. In the case of phenolic compounds, these biosensors are often based on the enzymatic oxidation by enzymes such as tyrosinase [32] or laccase [33]. Metal composites have been used to modify the working electrodes and to provide a large surface area for enzyme immobilization and improved surface charge transfer.
Peptides are oligomers and polymers that can be customized with highly controlled preparation methods, due to the variety of natural and synthetic amino acids available for synthesis. Peptides have been employed in biosensing due to their specificity, better chemical and conformational stability compared to antibodies [34], low cost and facile synthesis and modification protocols that allow customization for a wide variety of applications [35].
Microbial biosensors use microorganisms as a sensitive biological element. Their main advantage is the fact that, unlike molecule-based biosensors, they provide information on toxicity or bioavailability [36]. The microbes are usually genetically engineered by modifying their structure to serve as bio-receptors for the target molecule [37].