1000/1000
Hot
Most Recent

Membrane separation (MS) and electrochemical advanced oxidation process (EAOPs) are the important technologies in water pollution control. They can not only effectively remove pollutants in water, but also have the advantages of environmental friendliness, easy automation and low land occupation. However, both MS and EAOPs still have some problems to overcome, namely membrane fouling and high energy consumption. In recent years, many researchers proposed that the combination of the two technologies can overcome this problem. In this case, both of them can be used as a pretreatment for the other to achieve different purposes. When MS is prior to EAOPs, the purpose is to reduce the turbidity as well as to concentrate pollutants and salinity in order to weaken the mass transfer limitation and increase the conductivity. When setting MS after EAOPs, the purpose is to treat the concentrate to meet the discharge standard or permeate water to further improve the quality for reuse. The combination of MS and EAOPs offers a valuable way to implement them in the practical project of wastewater treatment which deserves more attention.
The use of MS in water reclamation plants (WRPs) has developed rapidly due to its ability to separate components with different sizes or physical/chemical properties; examples include particle filtration (PF), microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), forward osmosis (FO), membrane distillation (MD), and membrane bioreactors (MBRs) [1][2]. WRPs are constructed with the objective of reusing wastewater for different purposes, such as recreational, agricultural, or industrial purposes [3]. Thus, MS is often used as a tertiary treatment to produce high-quality water that is suitable for recycling or reclamation after removing organic contaminants, suspended solids, and other substrates by biological treatment [4]. However, MS often suffers from its own “Achilles heel”. During operation, the interception and accumulation of contaminants on the membrane surface or inside the membrane pores will result in membrane rejection and fouling [5], which inevitably deteriorates membrane performance and life [6]. For the treatment of most chemical–industrial wastewaters, setting EAOPs prior to MS has been regarded as a good idea and can considerably reduce membrane fouling due to the removal of organic contaminants in EAOPs.
In addition, MS is essentially a physical process of obstruction and concentration, which means that contaminants are not actually “degraded”. Therefore, it is impossible to achieve complete purification simply by MS when wastewater contains refractory organic pollutants. In contrast, EAOPs can decompose refractory organic compounds into biodegradable by-products or low molecular species [7]. For the past few years, EAOPs have been widely applied in WRPs to degrade organic contaminants such as pesticides [8], textile dyes [9][10], landfill leachate [11], pharmaceuticals [12], and explosive chemicals [13]. Although EAOPs are an effective method of addressing toxic contaminants, drawbacks remain due to their high energy consumption caused by the low efficiency of mass transfer. Notably, concentrate from wastewater treated by MS has a relatively high concentration, which can alleviate mass transfer limitations. Moreover, the electrolyte concentration increases during the MS process, which raises the whole system’s conductivity, leading to a significant reduction in energy consumption [14][15]. In this case, one way to overcome challenges by developing mature technologies for commercial applications could be to combine MS and EAOPs. Herein, MS and EAOPs stand as separate units to form a two-stage process of EAOPs-MS or MS-EAOPs. To date, many researchers have studied and reported many synergistic designs of such coupled processes for industrial wastewater treatment.
Figure 1 presents the combination strategy of MS and EAOPs; either MS or the EAOPs can serve as a pretreatment for the other. When EAOPs are applied before MS, the purpose is to degrade organic contaminants to relieve the treatment load of the membrane. When EAOPs are applied after MS, the aim is to deeply treat the membrane concentrate to meet the discharge standard or treat permeate water to further improve the quality for reuse [16].
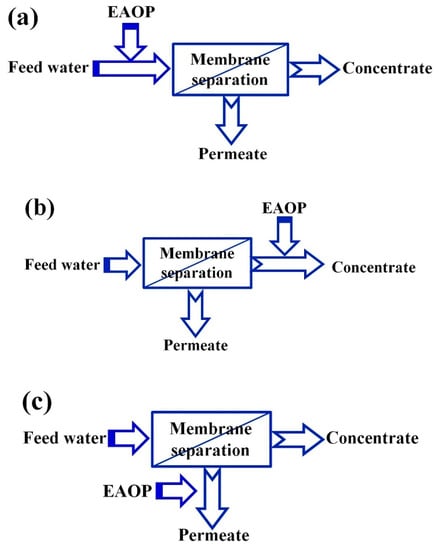
Figure 1. Coupling of membrane processes with electrochemical advanced oxidation processes (EAOPs, the two-stage processes): (a) Pre-treatment of feed; (b) Post-treatment of concentrate; (c) Advance treatment of permeate [3].
The combined process of EAOPs-MS appears promising for wastewater treatment due to its highly efficient removal of organics and salinity. Chen et al. [17] set up a single-cell electrochemical reactor with a PbO2 anode and arc-shaped transfer-flow membrane module in alternating sequence to treat wastewater from a textile dye house. Electrochemical oxidation (EO) and MS processes complemented each other, as EO effectively removed the chemical oxygen demand (COD) and chroma and MS nearly completely removed total suspended solids (TSS). Masid et al. [18]investigated the effectiveness of combined treatment processes of coagulation/flocculation (C/F), EO, and membrane processes for tackling the organic load in segregated chemical industry wastewater. Three different combined processes of UF-RO (CP-I), C/F-EO-UF-RO (CP-II), and C/F-EO1-EO2-UF-RO (CP-III) were investigated. The overall COD and total dissolved solids (TDS) removal efficiency was in the order CP-III (93% and 87%) > CP-II (84% and 85%) > CP-I (73% and 82%). These results showed that the EO process considerably reduced the organic load in the effluent, while a large part of TDS removal was attributed to the final RO treatment. Diogo et al. [19] established a combined electrochemical/membrane filtration process for the treatment of wastewater containing phenol and an azo dye (Acid Orange 7, AO7). A boron-doped diamond (BDD) electrode was used as the anode in the electrochemical treatment, followed by RO and NF membranes, which were used in the concentration step. Even though the COD removal in both cases was more than 95%, the combined process only targeted soluble organic pollutants, not suspended particulates.
The above research studied two-stage combination processes. However, with the development of EO and MS hybrid systems, an increasing number of studies have focused on single reactors. Xu et al. [20]reported interesting work on a single reactor where a mesh catalytic electrode was placed on the intercept side of an NF membrane to build a coupled system for dye wastewater treatment. Experiments showed that electroosmosis, electrophoresis, and EO effectively restrained membrane fouling and concentration polarization. Moreover, a high permeate flux was obtained under a relatively low cross flow velocity and pressure. This work provided a novel way to reduce the investment in equipment and the cost of membrane cleaning and replacement while reducing the operating pressure and area of the membrane. Another single reactor was designed by Juang et al. [21], who assembled an EO/MS hybrid system using a Ti/BDD electrode and ceramic membrane to remove Acid Yellow 36 (AY-36) in dye wastewater. Complete COD removal and a more than 90% reduction in turbidity and chroma were achieved. Scaling up laboratory systems to satisfy practical application needs is often associated with an increase in energy consumption, which is an urgent problem to solve. For this purpose, Madsen et al. [22] investigated the reduction in the energy consumption of EO when combining the process with MF, NF, and RO membranes and provided a realistic estimate of the energy consumption during treatment. The pesticide residue 2, 6-dichlorobenzamide (BAM) was used as the target pollutant. The results showed that membranes significantly reduced the energy consumption of the EO process. When using an RO membrane with a recovery flux of 90%, the energy consumption was 95% less (0.96 kWh m−3) than that of single EO treatment (18.5 kWh m−3). Subsequently, with the breakthroughs of the energy consumption and membrane fouling bottlenecks, the combined process of MS and EO at the laboratory scale and pilot scale has been extensively studied. Mameda et al. [23] designed a new membrane-electrode hybrid reactor (MEO) at the pilot scale as a tertiary treatment for secondary textile wastewater effluent with a particular focus on controlling membrane fouling. The MEO achieved substantial removal of color (50–90%), turbidity (> 90%), bacteria (>4 log), COD (13–31%), and 1,4-dioxane (≈25–53%). Furthermore, the pilot-scale test confirmed a substantial delay in membrane fouling (by more than 40 times) at a current density of 150 A m−3 compared to the control group. Du et al. [24] reported other interesting work using the PMS-assisted EO/electrolytic coagulation (EC) process as a pretreatment to improve water quality and reduce the fouling behavior of a combined ceramic UF process. Sulfamethazine (SMZ) and organic matter were degraded into small-molecule organics by sulfate radicals (SO42−) or hydroxyl radicals (·OH), which led to a significantly lower membrane fouling rate. In particular, this process could achieve stable permeation and retain better filtration performance in the case of high antibiotic and organic matter contents in real surface water treatment.
MS can also be used as a pretreatment process before EAOPs. The system can be divided into two configurations depending on whether the EAOPs are applied to treat the membrane permeate or concentrate. This technique has great significance for energy savings and environmental protection, as the treated permeate can be reused for production to reduce the consumption of raw water and the treated concentrate can be directly discharged to the environment.
For permeate treatment, a common example is to use UF or loose NF membranes to reduce water turbidity before the water enters EAOPs. Acosta-Santoyo et al. [25] reported a novel combined process with UF as a preconcentration stage followed by electrochemical degradation of oxyfluorfen with BDD anodes. The efficiency of oxyfluorfen degradation by EO increased with current density, while the degradation of total organic carbon (TOC) followed an opposite trend. According to their results, it would be worthwhile to use UF as a concentration stage for commercial formulations of nonpolar organochlorines due to the high rejection and flux. However, a concentration degree of 2.3 by UF is not enough to decrease the power consumption needed to remove oxyfluorfen by EO. This result could be attributed to the lower proportion of surfactant after the UF process, which hinders the production of persulfate. Mostafazadeh et al. [26] investigated the treatment and reusability of laundry wastewater using UF as the first step followed by EO. Under optimum conditions, UF could achieve 50%, 95%, 97%, and 75% rejection of COD, TSS, turbidity, and nonylphenol ethoxylate (NPEO3-17, respectively). Moreover, UF permeate treated by EO with a current density of 12 A and a treatment time of 45 min using a BDD anode and graphite cathode achieved a COD value lower than of 80 mg L−1, suggesting the potential possibility of reuse.
For concentrate treatment, NF and RO are often followed by EAOPs to deeply treat the concentrate prior to disposal. Many studies have proven that this technique exhibits excellent performance with several advantages, such as low energy consumption due to excellent conductivity after NF or RO and the possibility of indirect bulk oxidation through the electrogeneration of strong active chlorine oxidants [27][28]. Van Hege et al. [29][30] published a pioneering study that investigated the reduction of recalcitrant organic constituents as well as the removal of total ammonia nitrogen in RO retentate by electrochemical treatment using RuO2 and BDD electrodes. Analysis of the inorganic chlorinated species revealed that the oxidation mechanism was mainly due to the indirect oxidative action of electrogenerated hypochlorite. Chaplin et al. [31]reported that the electrochemical destruction of N-nitrosodimethylamine (NDMA) was achieved in RO concentrate (ROC) containing high concentrations of dissolved organic carbon (DOC) and hydroxyl radical scavengers. The destruction half-lives of 3.1 and 6.5 min indicated that a practical system for removing organic matter from ROC or other wastewaters may be feasible. Soriano et al. [32] investigated the treatment of NF concentrate by EO, and the system effectively achieved the removal and mineralization of perfluorohexanoic acid (PFHxA). The electrochemical degradation rate of PFHxA reached 98%, while the energy consumption (15.2 kWh m−3) was minimized by selecting an operation parameter of 50 A m−2. Kateb et al. [33] studied the mineralization and biodegradability enhancement of NF concentrate from landfill leachate by EAOPs. Their results showed that the most efficient treatment strategy appeared to be heterogeneous electro-Fenton (EF) at 4.2 mA cm−2 combined with anodic oxidation using a Ti4O7 anode (energy consumption = 0.11 kWh (g DOC) −1). However, it should be noted that enhanced nitrification is required because of the release of NH4+ from the mineralization of refractory organic nitrogen by EAOPs.