1000/1000
Hot
Most Recent

Siderophores are iron-complexing compounds synthesized by bacteria and fungi. They are low molecular weight compounds (500-1500 Daltons) possessing high affinity for iron(III). This entry reports an integrated computational/synthetic approach towards a rational development of peptide-based siderophores.
Hexadentate iron(III) specific catecholato ligands have been extensively studied [1], for instance there is a large range of natural hexadentate chelators, termed siderophores, which are used by microorganisms to scavenge iron from the environment [2]. In addition synthetic hexadentate catecholato chelators have been designed to compete with natural siderophores, resulting in antimicrobial properties [3]; to scavenge toxic metals from the environment, for instance plutonium, [4] and to form high affinity complexes of Gd4+, Ga3+, In3+ for medical imaging [5][6][7]. The synthesis of such hexadentate ligands can be complicated in so far as often the synthetic routes consist of over ten consecutive reactions and the deprotection of oligodentate ligands is frequently time consuming and difficult to achieve complete deprotection [8][9]. Both these characteristics tend to lead to low yields. Furthermore, the determination of iron(III) affinity constants and pKa values for tricatecholato chelators are difficult by virtue of their extremely high values [10]. Thus the ability to predict iron(III) affinity trends of a series of theoretical catecholato ligands is likely to facilitate such work. Raymond and colleagues have previously reported studies on prediction models to rationalise the design of hexadentate catecholato iron ligands [11].
In order to simplify synthetic routes, we have developed a solid-phase approach for the preparation of peptide-based tricatecholate containing peptides. Fmoc amino acids were adopted, together with the coupling agents PyOxP/DIPEA, or OXYMA/DIC. Peptide deprotection was achieved with 20% v/v piperidine in DMF. BBr3 was utilized for the multiple demethylation of 2,3-dimethoxybenzoic acid (DBA), followed by semi-preparative HPLC purification. In addition, to rationally design improved peptide-based ligands, we also report here the use of Density Function Theory (DFT) [12] to predict the structure of iron complexes. We reasoned that the degree of deviation from the ideal iron coordination sphere could provide an indication of the stability of such complexes. From structural considerations, the angle of an ideal octahedral iron(III) d2sp3 hybrid should be 180° [13]. The sum of the 3 transverse angles in a perfect hexagon will be 180 × 3 = 540°. This can only be achieved by monodentate ligands such as OH-. When bidentate ligands coordinate iron(III), for instance catechol, the angle provided by each ligand is less than 180° due to the restricted bite distance of the two oxygens; for catechol the angle is 167° and thus the sum of the three transverse angles is 501.9° [14]. This deviation from 540° will lead to a decreased ∆H for complexation as the overlap of the orbitals cannot be maximized. When 3 such bidentate units are joined together by a molecular spacer, such octahedron distortion may become even larger leading to a further decrease in the enthalpy of complexation. We have compared the DFT-determined structures of a range of iron(III) siderophore complexes with their corresponding X-ray diffraction derived structures and found excellent agreement [15].
Hereby we describe a rapid, efficient, and versatile solid-phase approach for the preparation of a small library of peptide-based tricatecholate containing peptides (Figure 1). A generic synthetic route for the preparation of a lysine-based tricatechol (3) is presented in Figure 2. NovaPEG Rink Amide resin (hereby used to prepare carboxy amidated peptides) was coupled with the orthogonally protected Fmoc-Lys(Mtt)-OH using OXYMA/DIC chemistry [16], followed by removal of the Fmoc group with 20% piperidine/DMF. The process can be repeated as many times as required, for instance three acylations can be conducted for the preparation of a hexadentate scaffold. Boc-Lys(Mtt)-OH was preferred as the amino-terminal amino acid since the Boc group could be simultaneously removed together with the cleavage of the peptide from the solid support under high TFA concentration conditions. Boc-Lys(Mtt)-OH can be coupled at the N-terminus just before a multiple N-methyltrityl- (Mtt-) side chain deprotection is required. N-terminal Fmoc-protected amino acid was employed whenever further chemistry was conducted at the amino-terminus site, such in the case of N-terminal acetylation, N-terminal further derivatization, or for the synthesis of the octadentate ligands, which is the subject of future investigations in our laboratory.
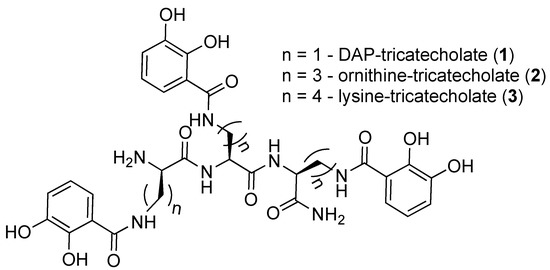
Figure 1. Structures of the synthesized tricatecholato-containing peptides.
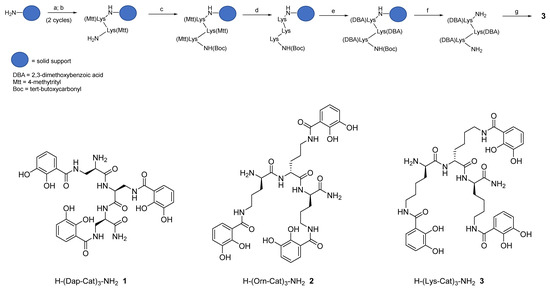
Figure 2. Schematic route for the synthesis of hexadentate peptide-chelator 3: a) Fmoc-Lys(Mtt)-OH, Oxyma, N,N’- diisopropylcarbodiimide (DIC), DMF; b) 20% piperidine/DMF; c) Boc-Lys(Mtt)-OH, Oxyma, DIC, DMF; d) 1% TFA/DCM; e) 2,3-dimethoxybenzoic acid (DBA), PyOxP/DIPEA, DMF; f) TFA; g) BBr3/DCM.
The above procedure was repeated for the preparation of tricatecholate-based peptides where the catechol groups were linked to the peptide scaffold via a diaminopropionic acid (DAP) and ornithine group for compounds (1 and 2), respectively (Figure 2). Accordingly, a “mixed amino acid” approach could be undertaken if mixtures of side arm lengths are desired, such is the case of H-(Lys-Cat)2(Dap-Cat)-NH2 (19) (Table 2, Figure 4).
Following solid-phase assembly of the scaffolding structure, selective removal of the Mtt group was achieved using 1% TFA/DCM. Subsequently, coupling with 2,3 dimethoxybenzoic acid (DBA) was most efficiently achieved using PyOxP/DIPEA in DMF [17], as in our hands the multiple acylation of DBA residues were incomplete using standard peptide coupling conditions. Following removal of the protected-catecholate (DBA) peptide from the solid support, demethylation of the DBA residues was accomplished under BBr3/DCM conditions. The crude compound was finally purified using semi-preparative reverse-phase (RP) HPLC-DAD-MS and further characterized via analytical RP-HPLC-DAD, HRMS, and 1H NMR (see supplementary material).
As mentioned above, the route is applicable for the assembly of “hybrid” chelators, where mixed arm catechols but also other chelator-types may be conjugated into the same scaffold. This can be achieved by selective deprotection of an orthogonal protecting group in the growing Fmoc-N-terminal peptide sequence followed by acylation with an appropriate carboxylic acid-containing chelator. Subsequently, the Fmoc-group is deprotected and the next Fmoc-amino acid coupled. The conjugation of another chelating unit can then be achieved by repeating one more time the above cycle. The preparation of a library of “hybrid” chelators is currently under investigation in our laboratory.
To predict improved synthetic siderophores that could be synthesized by our newly developed solid-phase method, we have investigated in-silico 5 siderophores, namely 4 – 8, together with 4 siderophore analogues 9 – 12. The two parameters, transverse angle sum and the roots mean square differences (RMSD) between the octahedral of oxygen atoms in the complex and a perfect octahedron (see Materials and Methods), were used to analyse the entire group of the 9 iron(III) complexes (Table 1).
Table 1. Siderophore analogues.
|
Ligand in FeL Complex |
Sum of Angles 2 |
RMSD 1 |
|
4 |
509.1 |
0.260 |
|
5 |
489.5 |
0.300 |
|
6 |
486.6 |
0.286 |
|
7 |
506.4 |
0.238 |
|
8 |
487.5 |
0.315 |
|
9 |
481.6 |
0.320 |
|
10 |
478.0 |
0.340 |
|
11 |
474.8 |
0.340 |
|
12 |
486.5 |
0.269 |
Comparison of the 9 compounds demonstrated that only two, bacillibactin (4) and corynebactin (7), were located in the vicinity of the “500/0.250” cutoff values (see Materials and Methods) (Figure 3). Both compounds possess relatively long linking arms between the central triester ring and the catechol function; namely 5 atoms. The remaining siderophores 5, 6 and 8 are clustered together and located well below the “500/0.250” cutoff values. Protochelin (5) has one short linking segment (2 atoms) and agrobactin (6) and vibriobactin (8) both possess rigid linking segments containing a heterocyclic ring. These factors clearly mitigate against the three coordinating catechol groups, forming an optimal stereochemistry around iron(III).
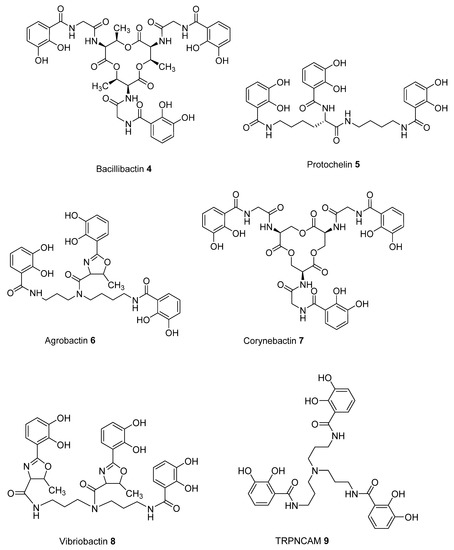
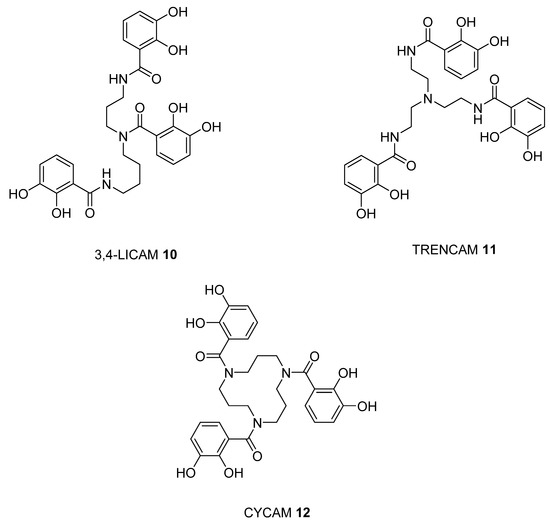
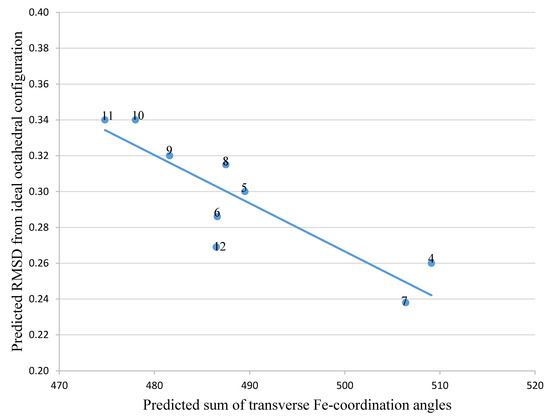
Figure 3. The relationship between predicted RMSD values and sum of transverse Fe-coordination angles for a series of siderophores and siderophore analogues.
With the catechol siderophore analogues (9, 10, 11, and 12), none are located close to the cutoff values, all the structures apparently providing a non-ideal geometry for optimal iron(III) coordination. Thus tripodal nitrogen based backbones form less suitable octahedral stereochemistry for the iron(III) binding site in comparison to amide-based siderophores, for instance 4 and 7.
We subsequently analysed the structures of ten hypothetical siderophore analogues which are based on various peptide linking groups (Table 2). Again, all these structures form a linear relationship between the two parameters, angle sum and RMSD (Figure 4). Two of the ligands (2 and 13) exceed the “500/0.250 cutoff” values but the rest do not. Based on this analysis, when three adjacent catechol-bearing arms are attached to a cyclic hexapeptide in the style of ferrichrome, namely 15 – 18, irrespective of the length of the linking group, a highly favorable iron(III) coordination site cannot be created and the two parameters, angle sum and RMSD, fall below the “500/0.250 cutoff” values (Figure 4). In contrast, when three catechol moieties are attached to a short linear peptide, 2 and 13, then a superior iron(III) binding site can be achieved (Table 2 and Figure 4). The stereochemistry of these molecules is critically dependent of the length of the amino acid chain, thus 1 and 4 methylene groups fail to yield an ideal iron(III) coordination site, whereas 2 and 3 generate such a site. Mixtures of side arm lengths are also capable of leading to compounds with a superior octahedral iron(III) binding site, thus although H-(Dap-Cat)3-NH2 (1) and H-(Lys-Cat)3-NH2 (3) fail to generate a high-affinity site for iron(III), H-(Lys-Cat)2(Dap-Cat)-NH2 (19) is capable of generating such a site (Table 2, Figure 4).
Table 2. Hypothetical catechol siderophore analogues.
|
Ligand in FeL complex |
Sum of angles2 |
RMSD1 |
|
1 |
489.4 |
0.270 |
|
2 |
502.9 |
0.230 |
|
3 |
466.2 |
0.450 |
|
13 |
501.0 |
0.230 |
|
14 |
480.2 |
0.307 |
|
15 |
488.5 |
0.309 |
|
16 |
482.9 |
0.291 |
|
17 |
494.3 |
0.280 |
|
18 |
477.4 |
0.350 |
|
19 |
498.9 |
0.230 |
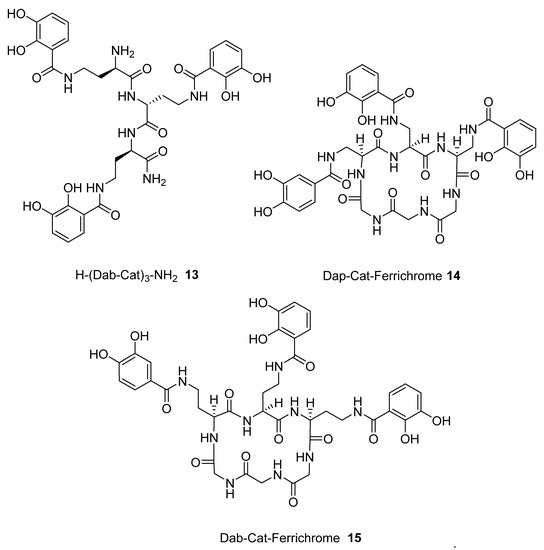
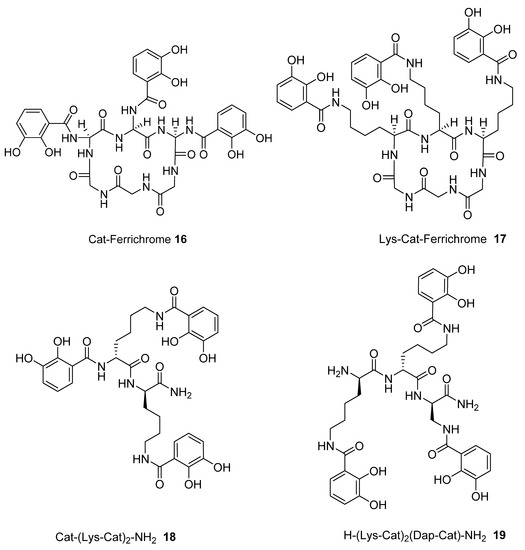
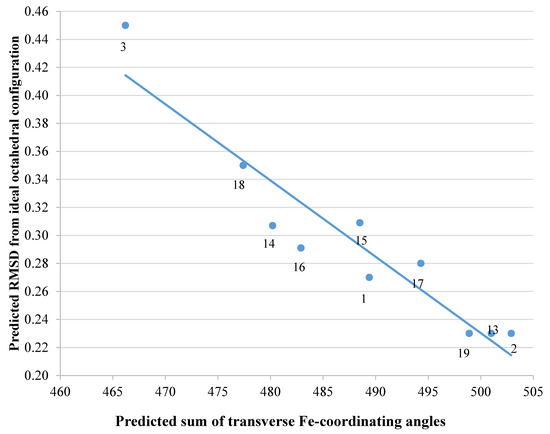
Figure 4. The relationship between predicted RMSD values and sum of transverse Fe-coordination angles for a series of peptide based tricatecholate ligands.
As indicated in the Introduction, the measurement of iron(III) affinity constants for hexadentate tricatechols is extremely difficult and we have been unable to determine accurate values for ligands (2 and 13) at the present time. However, we have used the method introduced by Ma et al. for the determination of pFe3+ values for multidentate ligands by fluorescence [18] and this analysis has preliminarily yielded a high pFe3+ value for the prototype ligand 2 (> 25). Further work is currently being conducted both for the preparation of siderophore analogue 13, and for the accurate determination of these ligands’ pFe3+ values to confirm our in-silico predictions.