1000/1000
Hot
Most Recent

Here, we report the creation of amphiphilic polymers based on the presence of a high-molecular weight hydrophobic poly(epichlorohydrin) backbone and hydrophobic pendant oligomeric poly(ethylene glycol) chains, whereby a combination of hydrophilicity and hydrophobicity induce folding of an individual polymer chain into a single-chain polymeric nanoparticle (SCPN) (1) at high solution concentrations, (2) without requiring addition of catalysts and additives, (3) in the absence of external stimuli, and (4) under ambient aqueous conditions. Thus, this approach can be an effective way to generate water-soluble SCPNs; this breakthrough in the development of SCPNs may enable a significant advance in enabling the broad application of biomedical fields.
Inspiration from nature has been the principal driving force behind the emergence of new functional polymers with unique and desirable physical characteristics that hold great potential for applications in various fields [1][2]. These SCPNs exhibit unique physical and chemical properties, including low fluid viscosities, low hydrodynamic volumes and large surface-to-volume ratios [3]. In general, the fabrication of SCPNs relies on folding/collapse and intramolecular crosslinking (either noncovalent complex [4][5][6], dynamic covalent linkage [7][8][9], or covalent bonding [10][11][12]) of a single polymer chain to produce discrete SCPNs. However, when the polymer is present at low concentrations in solution, the presence of active reactive groups/catalyst additives and additional processes (e.g., photoirradiation and heat treatment) are required to induce a conformational change in individual polymer chains from coils to particles via intrachain interactions or chemical linkages. Compared to natural protein folding processes, the fabrication of well-defined SCPNs remains relatively inefficient and ineffective due to the difficulty of promoting and guiding spontaneous formation of SCPNs in solution. Therefore, there is an urgent need to effectively and systematically improve the formation of synthetic SCPNs under different reaction conditions.
Water-soluble SCPNs have attracted significant attention in recent years due to their potential applications in various fields, such as catalysis [13][14][15], drug delivery [16][17][18] and bioimaging [19][20][21]. Current strategies for the synthesis of water-soluble SCPNs are mainly based on noncovalent interactions (intramolecular hydrogen bonds and metal-ligand interactions) [5][13][22][23][24][25] and photochemical reactions [26][11][27] in aqueous solution. However, these synthetic techniques remain limited to the structural design and fabrication of water-soluble SCPNs [26][28][3]. To overcome some limitations in synthetic SCPNs, it is necessary to establish a facile and efficient route for the preparation of water-soluble SCPNs with improved structural stability in aqueous media [29][30]. In our previous work, polyethylene glycol (PEG)-grafted amphiphilic polymers with high grafting densities facilitated polymeric self-assembly in water and efficiently induced phase separation between hydrophilic and hydrophobic segments to promote the formation of water-soluble micelles with a small diameter (<25 nm) [31]. This finding inspired us to further explore a simple and highly efficient route for the synthesis of water-soluble SCPNs. Thus, we boldly conjectured that the introduction of hydrophilic PEG chains into a high-molecular weight hydrophobic polymer backbone may significantly affect the polymer self-assembly process and effectively induce folding/collapse of the polymers into SCPNs in aqueous environments. This new strategy could be expected to greatly improve the formation and stability of SCPNs due to the possible presence of a large volume number of hydrophobic backbones and a combination of hydrophilic and hydrophobic interactions within the SCPN structure [31].
Herein, we provide a facile route for the preparation of functional amphiphilic polymers that spontaneously self-assemble into SCPNs in water with desirable physical properties. Water-soluble ultrahigh molecular weight amphiphilic polymers containing pendant PEG groups were successfully synthesized via a simple two-step process. The resulting polymers exhibit the excellent property characteristics of SCPNs in water, due to intramolecular chain-folding induced by strong amphiphilic interactions (Scheme 1). We exploited the amphiphilic effects within an ultrahigh molecular weight polymeric coil, whereby a combination of hydrophilicity and hydrophobicity induce folding/collapse of an individual polymer chain into an SCPN with unique physical properties and thus could potentially be used for biomedical applications.
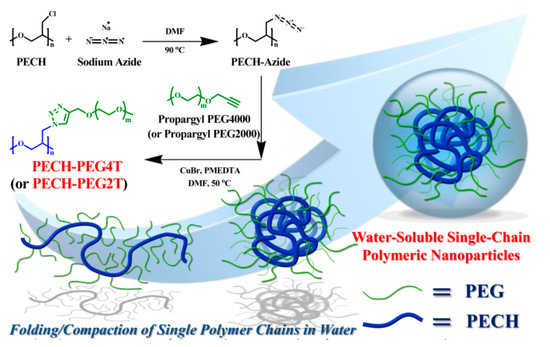
Scheme 1. Graphical representation of the synthetic procedures for PECH-PEG4T and PECH-PEG2T via self-assembly of isolated single-chain polymeric nanoparticles (SCPNs) through amphiphilic interactions between PEG-grafted poly(epichlorohydrin (PECH-PEG) polymers.