1000/1000
Hot
Most Recent

Lignin is an extremely abundant substance that has a chemical functionality that endows it with the necessary properties to be an attractive platform material for the new millennium. We must continue to explore and exploit its potentiality as a stand-alone material, raw feedstock for refining to chemicals, polymeric filler, and co-composite material. This review highlights hardwood-derived lignin, a little explored form of lignin, as a promising platform material.
Lignin is the main source of renewable aromatic structures on Earth [1][2]. The lignocellulose biorefinery is an interesting approach to maximize the value of lignin and its products. Lignin valorization for industrial applications depends on the development of novel technologies and approaches to overcome challenges mitigating conversion to valuable products [3]. Technical challenges include the need for efficient biomass pretreatment, lignin separation and purification technologies that can address lignin’s diverse structure and complex chemistry. Despite recent advances, there is currently limited conversion of hardwood kraft lignin (HWKL) products into bulk or fine chemicals. Difficulties with the production of value added products from lignin can partly be attributed to the highly complex and condensed nature of kraft lignin, the prevalence of highly recalcitrant lignin linkages/bonding motifs, and a high amount of polydispersity in addition to a considerable sulfur content [4].
The separation of lignin from the black liquor generated during the kraft pulping process can be achieved by acidification and ultrafiltration membranes. The acidification technique is based on the dissociation equilibria of weak acid groups which affects the solubility behavior of lignin-related chemical species. Membrane separation processes, on the other hand, can be efficient and cost-effective in many cases, however there are two key limitations: first, such processes get increasingly difficult to control as the concentration of the retained material increases. Second, the flux of permeate passing through a membrane tends to fall during continued usage due to such fouling phenomena as pore plugging and cake formation [5].
LignoBoost and LignoForce processes are the two main commercial technologies for precipitating lignin from black liquor [6], and both can be used to obtain hardwood kraft lignin. The LignoBoost process uses dissolved carbon dioxide (CO2) to decrease the pH of the process stream from ~13 to 10 (Figure 1). It is well known that when the BL is acidified, the phenoxide groups of the dissolved lignin become protonated and lignin solubility decreases, i.e., the lignin precipitates. After precipitation, the solids are separated by filtration, then subsequently re-suspended in water and sulfuric acid (H2SO4) to obtain a lower pH of ~2.5 to remove impurities [5].
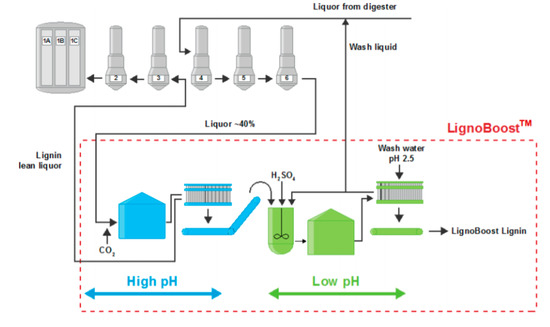
Figure 1. LignoBoost process by Stora Enso.
At pH values of less than 11, significant quantities of total reduced sulfur (TRS) compounds and other volatile sulfur species can be generated. Such compounds are strongly odorous with well-known negative effects on human health and other forms of life. Thus, LignoForce was created to address these issues [7]. LignoForce is a commercialized technology that first oxidizes the black liquor with O2 and then acidifies to pH ~ 9 with CO2 [8] (Figure 2).
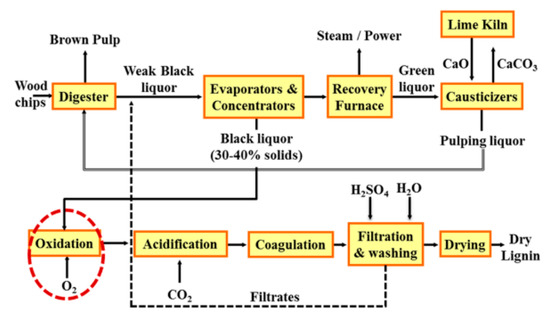
Figure 2. LignoForce System by FPInnovations and NORAM [7].
It is worth mentioning that lignin needs to be precipitated from pulping spent liquors effectively and selectively to have an economically feasible lignin production [8]. Unfortunately, acidic conditions used in lignin precipitation are known to cause some β-ether cleavage and lignin condensation [9]. Thus, the process conditions for lignin recovery from black liquor may interfere with its processing and use. The cleavage and condensation reactions are depicted in Figure 3.
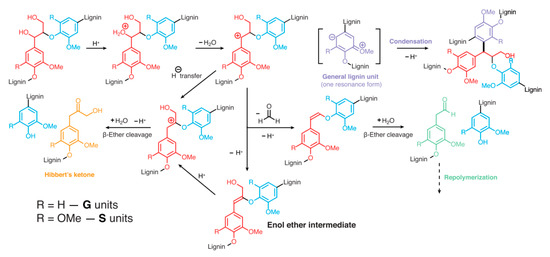
Figure 3. Lignin acidolysis and condensation routes [9].
The use of extracted lignins rather than whole biomass can claim an advantage in that the material can be fully dissolved in organic solvents, facilitating recovery and continuous processing [9] for diversification of products and hence value creation for the pulp and paper industry. However, it is important to mention that lignins can be extracted from the whole biomass. Lignins, in any form, are soluble in ionic liquids (ILs), which facilitates extraction from lignocellulose. The extraction can be performed with or without dissolution of the biomass [10]. ILs are considered green solvents due to their non-volatility and low flammability. Furthermore, ILs are not only used as solvents but also play an important part in catalytic cycles in pulping reactions [11]. However, ILs have a major shortcoming as they are much more expensive when compared to common and traditional solvents. Thus, recoverability of ILs should be explored and emphasized. Due to the π–π interaction between ILs and lignin, removal of lignin from ILs is proven to be a complex process and, therefore, requires multiple steps [12]. This makes the recycling and regeneration of ILs, particularly in extremely large volumes, equally cost inefficient [13].
Moreover, deep eutectic solvents (DESs) has been reported to completely isolate lignin from lignocellulosic biomass in a one-pot procedure [14]. DESs are green and inexpensive solvents, that have emerged at the beginning of this century to overcome the problems of ILs [15]. Similar to ILs, DESs have interesting properties including negligible volatility, non-flammability, and high conductivities [16].
Major research and development findings for the applications of hardwood kraft lignins are addressed within this section.
The interest in renewable energy sources (RES) has been increasing worldwide as evidenced by the massive interest in pellets and briquettes. These two materials are fuels manufactured from biomass. European countries consumed 50% of the global wood pellets in 2018 [17]. Furthermore, the United States manufactured 8.2 million tons in the same year; being the second largest producer in the world only surpassed by China. It should be noted that the market continues to grow due to high demand from overseas markets.
Direct addition of 6% (w/w) of HWKL to briquettes has been reported to positively influence apparent density, energy density, heating value, and mechanical resistance of the material [18]. Moreover, the addition of HWKL to pellets has been reported to be feasible. Improvements in physical and mechanical properties (density, mechanical durability, fines, and hardness) have been observed [19]. This study highlighted the importance of a lignin with low ash and moisture contents for briquette and pellet applications.
Although this application is still at the research level, the previously mentioned promising results point to the high potential of briquettes and pellets to supply part of the energy consumed around the world, with a vast potential utilization curve for hardwood kraft lignin without concomitant fractionation and/or modification.
Dispersant is a term generically used to describe surfactants, plasticizers, or emulsifiers, depending on the field of application. Healthcare, food, civil construction, and agriculture greatly benefit from dispersants that enable the mixing of immiscible liquid phases and enhance the stability of particle suspensions. Dispersants lower the interfacial tension between immiscible liquids, as well as increasing the repulsive forces between suspended particles and prevent settling and aggregation of phases, thus improving technical properties of multiphase systems such as rheology, lifetime, and function [20].
As discussed in previous sections, kraft lignins are not water-soluble. Thus, to use HWKL as a dispersant, modification is required. A study of carboxymethylated hardwood kraft lignin shows that it can successfully be used as a dispersant for clay suspensions [21]. The researchers added that it could potentially be applied in pesticide formulations, ceramic suspensions, and as a cement admixture. The optimal conditions for carboxymethylation were 1.5 M NaOH concentration, a 3 mol/mol sodium chloroacetate (SCA)/lignin ratio, 40 °C, 4 h and 16.7 g/L lignin concentration. In addition, this modified lignin had a charge density and carboxylate group of 1.8 meq/g and 1.68 mmol/g, respectively.
In another study, sulfometylated HWKL was produced with formaldehyde and sodium sulfite under alkali conditions. The optimum conditions for the lignin modification were 0.5 M NaOH(aq), 0.9 mol/mol sodium hydroxymethyl sulfonate/lignin at 100 °C for 3 h, and 20 g/L lignin concentration [22]. It was shown that the modified lignin had a charge density of −1.60 meq/g and sulfonate group content of 1.48 mmol/g. The sulfomethylated lignin was used as a cement dispersant, and the dispersibility of cement was increased from 60 to 155 mm by adding 1.2 wt% of modified lignin to cement. The researchers also evaluated the addition of unmodified lignin, which did not change the dispersibility of cement.
The majority of the industrially used dispersants are synthesized from non-renewable precursors and are not biodegradable, raising concerns over their sustainability [20]. Therefore, the development of lignin-based dispersants is an attractive solution. Moreover, laboratory experiments have already shown that its manufacturing from modified HWKL is feasible.
Lignin presents a good capacity to adsorb heavy metal ions because it possesses two types of acidic sites (carboxyl and phenol groups) that participate in the sorption mechanism. Thus, ion exchange using lignin has been studied as a potential low cost method for wastewater purification [1][23]. Eucalyptus kraft lignin has been studied for the removal of Cu(II) and Cd(II) from water/wastewaters in single and multi-component systems [23]. The researchers highlighted a superior performance of HWKL compared to most adsorbents, carbons, and biosorbents currently utilized. It was also mentioned that hardwood kraft lignin as an adsorbent is not commercial yet, however laboratory results show that it can be applied for development of large-scale systems.
Besides ion-exchange, lignin derivatives can efficiently capture metal ions through chelation and electrostatic interactions. A review paper about lignin application as an adsorbent of heavy metal states that lignin can be modified by physical/chemical methods to fabricate desirable adsorbents with good sorption capacity and selectivity for the target metals [24]. The researchers also mentioned that lignin-based materials have shown outstanding sorption for metals such as toxic metals (Hg), precious metals (Ag), and metal anions (Cr). Furthermore, it was recommended that specific emphasis should be placed on the lignin modification to design and develop advanced lignin-based adsorbents.
Hydrogels are three-dimensional polymeric networks formed from cross-linked hydrophilic polymers. They are insoluble and capable of retaining a large amount of water in their swollen state. They are typically used for contact lenses, hygiene products, wound dressings, drug delivery and tissue engineering.
Hydrogels synthesis through radical polymerization of hardwood kraft lignin, N-isopropylacrylamide, and N,N′-methylenebisacrylamide is shown in Figure 4.
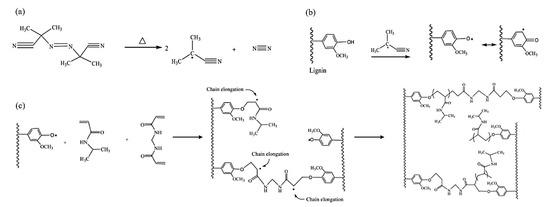
Figure 4. Radical polymerization reaction for lignin-based hydrogel production: (a) decomposition of Azobisisobutyronitrile (AIBN) initiator, (b) formation of phenoxy radicals, and (c) cross-linking reaction [25].
The reactions involved in the production of lignin-based hydrogels are well described elsewhere [25]. The results of the study showed that lignin-based hydrogels exhibited less swelling affinity because they possessed a reduced surface area and a less porous structure than synthetic hydrogels. On the other hand, they were reported to be more thermally stable. The incorporation of lignin generated a less cross-linked hydrogel which tended to increase the rigidity and rheological stability of the hydrogel. It was also stated that, when compared to synthetic hydrogels, lignin-based hydrogels exhibited less elastic behavior as temperature increased. This is the only study dealing with hardwood kraft lignin in lignin-based hydrogels.
CF are high-strength light-weight materials and their application in composites takes advantage of their strength, stiffness, low weight, fatigue characteristics, lack of corrosion and heat insulation [26]. The main applications of CFs are in construction, electronics, transportation, and aviation. Currently, carbon fibers are manufactured with polyacrylonitrile (PAN) and pitch, two non-renewable materials.
One of the key driving forces for the promotion of the CFs market is the potential for lightweight automobiles. However, the high cost (~ $35/kg) of CFs can inhibit their utilization for commercial applications [27]. Lignin-based carbon fibers with their low-cost and sustainability appeal characterize a good alternative for the segment [28]. Furthermore, lignin is expected to offer additional benefits to CF, such as elimination of toxic substances involved in the preparation [29], lower melting temperature and faster stabilization [30] when compared with PAN- and pitch-based CFs.
To obtain lignin-derived CF, isolated lignin is first processed into fibers by extruding filaments from a melt or solvent swollen gel (spinning), and then the spun fibers are thermally stabilized in air where the lignin fiber is oxidized (stabilization). Afterwards, the fibers are subjected to pyrolysis under nitrogen or inert atmosphere, where fibers become carbonized through the elimination of hydrocarbon volatiles, their oxidized derivatives, carbon monoxide, carbon dioxide, and moisture [28]. Figure 5 provides a model workflow for the preparation of carbon fibers from lignin.
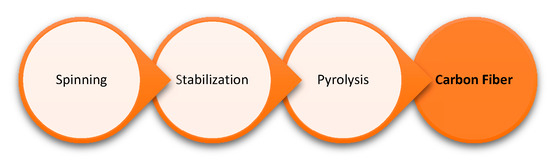
Figure 5. Workflow for the use of lignin as a precursor for carbon fiber.
CF from hardwood kraft lignin with mechanical properties suitable for general performance grades have been reported [31]. It was shown that thermally pretreating the lignin to remove volatile contaminants disrupts fiber integrity during subsequent thermal spinning and decreases hydroxyl content and subsequent intermolecular interactions by condensing the lignin aromatic nuclei.
The spinnability of lignin appears to be highly dependent on its structure. Hardwood lignins, whose structure is rather linear, can be melt spun without any additive [1] [31]. Blending HWKL with poly(ethyleneoxide) (PEO) produced miscible polymer blends which facilitated thermal spinning [31]. Furthermore, SWKL is reported to have spinning difficulties that can be overcome by addition of HWKL permeate as a softening agent [32].
It has been reported that HWKL can also be successfully transformed into CF by blending it with synthetic polymers such as poly(ethylene terephthalate) (PET) and poly(ethylene oxide) (PP), especially with the former [33]. Both systems were easily spun into fibers and blend composition affected surface morphology of the carbon fibers. Immiscible lignin–PP fibers resulted in a hollow and/or porous carbon fiber, whereas carbon fiber produced from miscible lignin–PET fibers tended to display a smooth surface.
Production of CF from a HWKL copolymer with PAN has also been reported. The resulting copolymer was confirmed by a Fourier transform infrared (FTIR), 13C, and 1H nuclear magnetic resonance (NMR) spectroscopy, showing the presence of the C≡N group fromPAN co-eluting with ether, hydroxyl, and aromatic groups that are attributed to lignin. The average tensile strength of the CF was 2.41 gf/den, a tensile strain of 11.04%, and a modulus of 22.92 gf/den [34].
Stabilized hardwood and softwood kraft lignin CF have shown a skin-core structure similar to fibers made from pitch [35]. Moreover, pore creation in immiscible polymer blends of hardwood kraft lignin and PP occurs by a two-step process: oxidative degradation of the PP component followed by pyrolysis gasification of residual PP related components. Gasification is the main factor for pore growth. The internal surface area of the lignin-based CF (499 m2/g) was lower than that for commercial activated carbons (745 m2/g) [36]. However, the researchers assure that relatively simple processes, such as steam activation, could effectively activate these porous lignin carbon fibers and make them suitable for commercial applications.
Finally, a recent article demonstrated the development of activated carbon fiber electrodes produced from HWKL to fabricate electric double layer capacitors (EDLCs) with high energy and power densities using an IL electrolyte [37]. A mixture solution of HWKL, polyethylene glycol as a sacrificial polymer, and hexamethylenetetramine as a crosslinker in dimethylformamide/acetic acid (6/4) was electrospun, and the obtained fibers were easily thermostabilized, followed by carbonization and steam activation to yield activated CF.
Antioxidant properties of HWKL have been determined by a DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical assay. This technique is based on electron-transfer that produces a violet solution in ethanol. The radical scavenger activity is expressed in terms of the number of antioxidants necessary to decrease the initial DPPH absorbance by 50% (IC50). The inhibitory effect of HWKL samples was ~8.4 µg/mL, whereas the IC50 for commercial antioxidant butylated hydroxytoluene (BHT), ascorbic acid, and Trolox was 13.3 µg/mL, 2.9 µg/mL, and 3.4 µg/mL, respectively [38], which shows the great antioxidant capacity of HWKL.
Overall, antioxidant activity increases with the phenolic hydroxyl content because they can scavenge free radicals, which are reduced with aliphatic hydroxyl content. Lignin with lower molecular mass and narrower molecular weight distribution seems to be beneficial [39], which also show the great potential of hardwood kraft lignins.
Another effort evaluated the antioxidant activity of HWKL by the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assay, which measures the relative ability of antioxidants to scavenge the ABTS generated in aqueous phase. The HWKL could oxidize ABTS to ABTS+ due to its reducing potential, which resulted in a color change (blue to green) [40].
Kraft lignins have shown potential to act as antioxidant for food, cosmetic and pharmaceutical industries instead of BHT, a synthetic resource. Lignin as a cosmetic or pharmaceutical product, is still not regulated because studies dealing with the safety of its use in humans are needed [38]. Recently, hardwood lignin nanoparticles were reported to enhance sunscreen performance. The best formulation had a UV transmittance of only 0.5–3.8% over the entire UVA−UVB region compared to 2.7–51.1% of a commercial SPF 15 sunscreen [41].
Lignin valorization in solvent systems to produce renewable aromatic chemicals has attracted great attention during recent years. The methodologies to obtain these compounds can be categorized as hydrolysis, hydrogenolysis, oxidation, and a two-step lignin depolymerization. Moreover, catalysis is a promising technique for lignin depolymerization to specific products [42].
Benzene, toluene, and xylene (BTX) and phenols are high-value chemicals that can be more sustainably sourced from lignin than from fossil–based resources [43]. BTX is the precursor for a series of materials such as resins, nylon fibers, polyurethane and polyester; thus, production of BTX from lignin could expand the utilization of lignin–based materials. It should be noted that when targeting chemicals from lignin, a key objective during the fractionation and depolymerization stage is to minimize lignin condensation as stated earlier in this review [44].
Other aromatic compounds that can be produced from lignin are syringaldehyde and vanillin. Eucalyptus and Northern European HWKL have been investigated to produce these compounds by oxidation with O2 in alkaline medium. The total yield of syringaldehyde was 14%, whereas for vanillin it was 16% [45]. In another study of oxygen oxidation of eucalyptus kraft lignin, under optimum conditions, only a reduced number of phenolic aldehydes (4%) was obtained. In contrast, when in the presence of catalysts, the yield could be increased to 14% with nitrobenzene and to 8% with CuO [46]. This was attributed to the low yield of transformation of the lignin/lignin oxidation products into low molecular weight acids.
Vanillin, the highest volume aroma chemical produced worldwide, is produced from a variety of sources, namely oil (85%), woody biomass (15%), and orchid pods (<1%). Around 20,000 tons of vanillin are produced per year, 15% of which comes from lignin (around 3000 tons/y) [47]. Lignosulfonates are the main sources for its production; however, kraft lignin could also be used for this purpose. The use of lignin by chemistry and polymer industries represents an important field of research with major issues in terms of scientific, economic, and environmental points of view and it seems justified that lignin will become a promising renewable aromatic resource in subsequent years [48]. Syringaldehyde is another promising aromatic aldehyde that possesses worthy bioactive properties which can be used in pharmaceuticals, food, cosmetics, textiles, pulp and paper industries, and biological control applications.
Lignin has been added to several polymers for the express purpose of potentially providing valuable new composite properties. Because of their large number of polar functional groups, lignin molecules interact strongly with each other. Most polymers are immiscible with lignin because of weaker interactions between lignin and the matrix polymer than among lignin molecules. Therefore, competitive interactions determine the structure and properties of blends and composites [49].
The miscibility of synthetic polymers such as poly(ethyleneoxide) (PEO), poly(ethylene terephthalate) (PET) and poly(vinyl alcohol) (PVA) with HWKL has been investigated. Miscible blends were observed in lignin/PEO and lignin/PET blends, while immiscible blends were found in lignin/PP and lignin/PVA blends [50]. The former polymers possess functional groups capable of interacting with lignin through secondary forces-bonding whereas the latter do not (Figure 6).
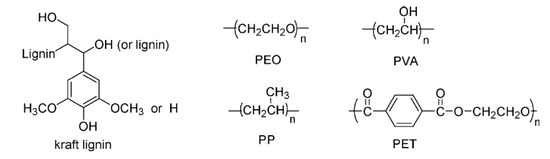
Figure 6. Structural representations of the various polymeric materials that can be blended with lignin [50].
A possible approach towards lignin valorization is using it as a component in plastics. The development of lignin-based thermoplastics relies on altering the viscoelastic properties of lignin through chemical modification or polymer blending [50]. The latter is a convenient method to develop products with desirable properties. The chemical and physical properties of the blends/composites are dependent on monomer type(s), molecular weight, and distribution and composition of the respective polymers [2][50].
Carbon-neutral thermoplastics have been successfully prepared by copolymerization of modified HWKL (oak variants) and dicarboxy-terminated polybutadiene (PBD-(COOH)2). Modified lignin (either by fractionation with methanol or by formaldehyde crosslinking) showed high molecular weight which facilitated preparation of free-standing films of lignin-based thermoplastic [51]. Additionally, it enabled formation of a more continuous network with telechelic polybutadiene, whereas the very broad molecular weight distribution of unmodified lignin formed a poorly networked structure.
Lignin-based thermoplastic miscible blends have been studied using HWKL and PEO. Incorporation of small amounts of PEO (5–10% w/w) sufficiently disrupts the lignin supramacromolecular complexes leading to enhanced physical properties. Increasing PEO incorporation further disrupts the lignin structure and the observed physical properties become more influenced by the PEO component [2].
It has been reported that olefinic thermoplastic polymer compositions comprising at least one polyolefin and eucalyptus kraft lignin, can successfully be manufactured [52]. The patent assures increase in the following properties: flow index (MFI), thermo-oxidative resistance (OIT-oxidative induction time), heat deflection temperature (HDT), stiffness (elasticity module), breaking strength, and flexural strength. In addition, the material maintains hardness and tensile strength measured at the outflow.
Blends of eucalyptus kraft lignin and PBAT (a biodegradable polyester, produced by BASF, and based on the monomers 1,4-butanediol, adipic acid, and terephthalic acid) have been studied. The study showed that the addition of up to 20% of lignin generated miscible or partially miscible structured blends, where lignin acted as a lubricant. Furthermore, the bends reached the technical requirements needed for sustainable solutions to rigid plastic apparatus in the agricultural segment, such as seedling tubes [53].
The intermolecular interactions of HWKL and PVA blend fibers prepared by thermal extrusion have been studied [54]. Although the blend is immiscible (presents two distinct Tg’s) some of the lignin was closely associated with the PVA in the PVA-rich phase. FT-IR analysis confirmed the formation of a relatively strong hydrogen bond between the hydroxyl groups of the short-chain PVA and lignin.
Petroleum-based polyol was replaced with hardwood kraft lignin preparing rigid polyurethane foam. The prepared foams contained from 9% to 28% (w/w) HKL [55]. The addition of lignin reduced the density of the foams, which is desirable if the foam is used as packing or insulation material. Furthermore, the authors reported that the majority of the kraft lignin was chemically crosslinked and the foams had satisfactory structure and strength up to 23% (w/w) addition when a chain extender was applied.
A polyurethane–lignin copolymer has been produced by step-growth polymerization of modified eucalyptus kraft lignin with isocyanate and doped by multi-wall carbon nanotubes (MWCNTs) [56][57][58]. Lignin possesses ion-exchange properties due to the presence of a variety of functional groups, which makes it an attractive active substance for chemical sensing. Co-polymerization allows the fixing of lignin inside a polymer matrix, ensuring high stability of the resulting material [56]. Furthermore, the electrical conductivity and impedance spectroscopy measurements revealed that the interaction between carbon nanotubes and lignin molecules in the polymer enhances its electrical conductivity [57].
Lignin fractions, having different molecular weights and varied chemical structures from HWKL (Eucalyptus grandis), were incorporated into a tunicate cellulose nanofibers (CNF)–starch mixture to prepare 100% bio-based composite films. In general, the addition of lignin led to a decrease in thermal stability and tensile strain and an increase in the Young’s modulus of the composite film [59]. Furthermore, some aggregates were observed in the composite films which may explain the lower tensile stress. The researchers pointed out that hardwood lignin with lower molecular weight and polydispersity reinforced the film structure.
The utilization of lignin in polyolefin blends is of growing research interest. However, the low compatibility of lignin and polyolefins restrains a satisfying blend production and leads to poor mechanical properties. It has been reported that it is possible to overcome these issues by modifying lignin prior to its incorporation into polymers to reduce its polarity [60]. The researchers performed esterification of eucalyptus kraft lignin and subsequently blended it with PE at a weight ratio of 1:1. They found a reduction of volume swelling and weight gain when modified lignin was used, which can be related to the esterification of hydroxyl groups as well as a significant reduction of sugar and ash contamination. Moreover, they observed that the elongation at maximum strength decreased to 22% (εM = 11%), while for other technical lignins the decrease was of ~10%. Highest tensile strengths were observed for the hardwood blends (17 N/mm2).
Copolymerization of N-isopropylacrylamide (NIPAM) with HWKL has been achieved by atomic transfer radical polymerization using a selectively modified lignin-based macroinitiator as shown in Figure 7. The thermal decomposition temperature of the lignin-polyNIPAM copolymers are reported to significantly increase with increasing degrees of polymerization of NIPAM. The solubility of the lignin–polyNIPAM copolymers in water was dependent on copolymer structure. In both the water-soluble and suspended copolymers, at temperatures above 32°C, the component has been reported to undergo hydrophilic-to-hydrophobic transition, resulting in precipitation of the copolymer [61].
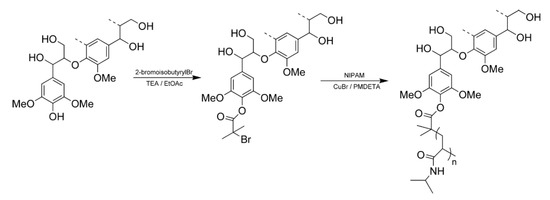
Figure 7. Scheme for the Preparation of Lignin-g-polyN-isopropylacrylamide (NIPAM) Copolymers [61].
Finally, lignin and acrylonitrile butadiene (ABS) blends and composites have received great attention recently. ABS is a very popular polymer material as shown by its widespread use as a thermoplastic resin in the automotive, marine, home appliance, toys, and other industries. Lignin is generally incompatible with ABS polymers, as shown by large phase separation domains with poor interfacial adhesion within the ABS matrix. This unfortunately leads to significant reductions in the strength and ductility of the resulting composite/blend, thereby limiting practical utility. A recent patent has addressed those issues and claims that by adding a compatibilizing agent, any lignin type, including HWKL, could be added to ABS plastics to provide increased stiffness and reduce cost [62]. The inventors used compatibilizing agents such as polyalkylene oxide, polyvinyl alcohol, polyvinyl acetate, ethylene vinylacetate copolymer, styrene-maleic copolymers, nitrile rubber, and others to assist in the dispersion and/or distribution and/or miscibility of lignin and ABS.
Due to lignin’s high-energy density and intrinsic aromatic based structure, this biomaterial is an ideal renewable feedstock that has tremendous potential in defining modern biorefinery. Lignin shows great potential to produce fuels, value-added chemicals, and functional materials, and ultimately reduce the environmental impact of their production. Although several studies have focused on converting lignin to valuable products, only a few of these efforts are commercially profitable, mainly because of low yields and low quality of the final products [3]. It is unfortunate that most lignin work deals with softwood kraft lignin, while hardwoods have been neglected. Because HWKL has a very different structure to SWKL, the knowledge acquired from the latter species cannot always be applied to the former. Thus, the intent of the present review was to provide a good basis for its possible valorization by overviewing the HWKL structure and properties. By appropriate understanding of the concepts in this review, it is hoped that utilization of this vast available and cheap aromatic source will be expanded to promote a firmer footing for a growing biorefinery.