1000/1000
Hot
Most Recent

The Retinoblastoma protein (pRb) is a key cell cycle regulator conserved in a wide variety of organisms. Experimental analysis of pRb’s functions in animals and plants has revealed that this protein participates in cell proliferation and differentiation processes. In addition, pRb in animals and its orthologs in plants (RBR), are part of highly conserved protein complexes which suggest the possibility that analogies exist not only between functions carried out by pRb orthologs themselves, but also in the structure and roles of the protein networks where these proteins are involved.
In humans, Retinoblastoma susceptibility gene is a member of a small gene family that includes RB1 (p105/pRb), RBl1 (p107/pRBL1), and RBl2 (p130/pRBL2), whose protein structure are very similar, and that share some overlapping functions [1][2][3].
The human Retinoblastoma protein (pRb) consists of 928 amino acids and includes three distinctive domains: the N-terminal structural domain (RbN), the so-called “pocket” (RbP) domain, the C-terminal domain (RbC), and the non-structured regions between them (Figure 1A). The pocket domain includes two highly conserved subdomains (A and B) called cyclin folds, which are formed by two structural nuclei, each conformed by three helix bundles with two additional helices packing at the sides in each one. These subdomains are required to mediate interactions with other proteins like several oncoproteins and transcription factors (TFs) [4][5][6][7]. According to current interaction databases 322 proteins interact with human pRb, the E2F TFs being the best characterized ones (Figure 1A) [8][9]. Many of the pRb-interacting proteins contain the motif ‘LxCxE’ (Leu-X-Cys-X-Glu where X stands for any amino acid), essential to bind with the Pocket B subdomain of pRb (Figure 1A).
In total, human pRb has 14 phosphorylation sites [7][10], two acetylation sites [11][12], six methylation sites [13][14][15], and it can also be modified through ubiquitination or sumoylation [16][17][18] (Figure 1B). Studies on the structure of pRb have revealed that phosphorylation changes pRb structure and promotes new interactions with other proteins.
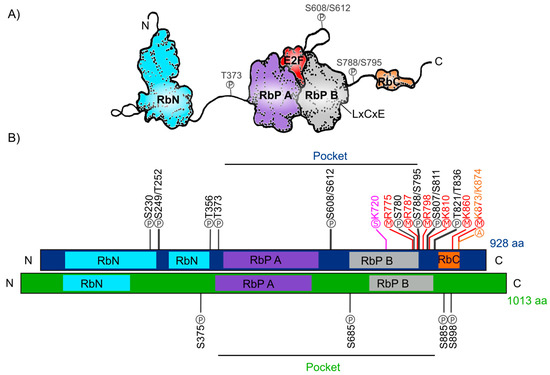
Figure 1. Retinoblastoma protein structure. (A) Representation of the human Rb protein structure with the domains RbN (blue), Pocket (RbP), with the RbP A (purple) and B (grey) subdomains interacting with an E2F TF (red), the RbC domain (orange) and the inter-domains (black lines) are also shown. The “P” inside a circle represents three examples of phosphorylation sites that change the structure of the protein. The position of the LxCxE cleft that allows Retinoblastoma protein (pRb) to interact with different proteins is also shown. (B) Comparison of the domains of human Rb protein (blue foreground) and Arabidopsis RBR protein (green foreground) and their reported post-translational modifications. Phosphorylation sites (P) are shown in black, methylation sites (M) in red, acetylation sites (A) in orange, and sumoylation sites (S) in pink.
The RB ortholog in plants was identified approximately a decade after the animal gene was discovered: RB1 gene was first described in humans between 1986 and 1989 [19][20][21] and the first plant orthologous RB1 cDNA (RBR), was first identified and cloned from maize in 1994 (ZmRBR) [22][23][24], then in tobacco (NtRBR) [25] and then in Arabidopsis (AtRBR) [26]. Afterwards, there have been numerous reports characterizing RBR orthologs in different plant species. Intriguingly, monocotyledons seem to have various RBR paralogs while dicotyledons have only one [27]. As a dicot plant, Arabidopsis carries a single copy of the RBR gene displaying ≈35% sequence similarity within the pocket domain with respect to the human pRb [28][29][30]. The Arabidopsis RBR (AtRBR) protein contains 1013 amino acids with the same modular structure of human pRb with putative phosphorylation residues. The role of four of them, located in the protein’s inter-domains, have been experimentally tested (Figure 1B) [31][32][33].
pRb hypophosphorylated acts as a negative regulator of cell cycle progression through its interaction with the E2F proteins. The heterodimer keeps the chromatin in a closed conformation in the regulatory regions of E2F-regulated genes [34][35]. When the pRb/E2F interaction is disrupted by loss or reduction of pRb, a high rate of cell proliferation is observed, and this generally triggers cancer [36]. pRb phosphorylation by cyclin/CDKs (cyclin-dependent kinases) complexes changes the pRb protein structure and its interactions with other proteins, inducing the release of E2F. For instance, this occurs in human cells when cyclin type D or E (CYCD/E) associate with cyclin kinases 4 or 2 (CDK4/2), respectively [34][37][38]. When pRb phosphorylation is altered, for example by overexpression of CYCDs, or by a miss regulation of CDKs, or by a disruption of the LxCxE-binding function the cell cycle is altered [39][40][41].
In humans, pRBL1 and pRBL2 participate in the repression of genes when cells are in the G0 quiescent state, through a complex called DREAM (DP-Rb-E2F-MuvB), that coordinates the repression of genes during quiescence and also the periodic gene expression during the G1/S and G2/M transitions [42][43]. DREAM is a multimeric protein complex that in humans is composed of DP (DP1-DP3), pRBL1 or pRBL2, E2F proteins (E2F4 and E2F5), and the subcomplex MuvB (Multi vulval class B). The MuvB subcomplex acts as a repressor when it is part of the DREAM complex, and is composed of LIN (LIN9, LIN37, LIN52, LIN54) and RBBP4 proteins. When pRb is phosphorylated, the DREAM complex is disassembled and the MuvB subcomplex can associate with TFs such as B-Myb (Myb type) and Fox-M1 (Forkhead box protein M 1), to promote the regulation of gene expression and the transition from quiescence to proliferation [42][44][45].
At about the same time that the pRb ortholog in plants was discovered, homologs of other components of the animal’s cell cycle regulatory machinery were identified and characterized in corn, as well as in Arabidopsis and Medicago sativa (alfalfa) [46][47][48][49][50]. In these plants, it was shown that the phosphorylation of RBR by the CDKA/CYCD protein complex regulates cell cycle progression, as it occurs in humans [51][52].
The DREAM complex also appears to be conserved in plants [53][54]. In addition to the presence of AtRBR, E2Fs/DPs, a MuvB-like complex has also been found that contains ALY2/3 (orthologs of LIN9) and TCX5 that is part of the TSO1-like family members (orthologs of LIN54) [55][56]. Furthermore, MYB3R, a transcription factor of the Myb family, has a protein structure resembling the DNA binding domain of B-Myb, which in humans forms part of the MuvB complex. These plant’s MYB3Rs also participate in the regulation of the G2/M transition as follows: MYB3R3 associates with E2Fc and AtRBR to repress G2/M genes, while MYB3R4 associates with E2Fb and AtRBR to activate G2/M genes [57][58][59].
The formation of any organ relies on two different but interlinked cellular processes: cell proliferation and cell differentiation. Proliferation produces all the cells that later will acquire fates, specialized functions, and morphologies through differential gene expression during cell differentiation [60][61]. pRb has been widely studied in proliferation and, recently, its participation in many different animal differentiation processes in the eye, brain, peripheral nervous system, muscle, liver, placenta, lung, cerebellum, pituitary gland, and heart has been elucidated (Figure 2A) [62][63][64][65][66][67].
Similar to pRb in animals, RBR participates in differentiation processes in the root and shoot meristems, the vascular system, leaves, stomas, and trichomes tissues in plants (Figure 2A) [68][69][70][71]. In Arabidopsis, AtRBR functions as a negative regulator of primary root development; its downregulation leads to longer roots with larger meristems whereas its overexpression results in shorter roots with smaller meristems [72].
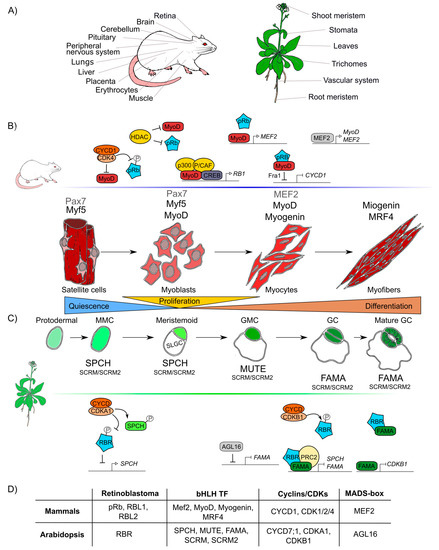
Figure 2. Retinoblastoma proteins are involved in cell differentiation in mammals and plants. (A) Mammals’ and plants’ organs whose differentiation depends on pRb/RBR. (B) Sequential steps of skeletal muscle differentiation in mammals. Shown are pRb interactions and bHLHs-family proteins (Mef2, MyoD, Myogenin, MRF4), involved in muscle quiescence maintenance, proliferation, and differentiation. (C) Sequential steps of guard cells differentiation in Arabidopsis. Shown are RBR interactions and bHLHs-family proteins (SPCH, MUTE, FAMA, SCRM, SCRM2) involved in quiescence maintenance, proliferation, and differentiation of guard cells. (D) Correlations between components involved in muscle and guard cell development in mammals and Arabidopsis, respectively.
It is difficult to compare the roles of pRb and RBR during differentiation due to lack of information and because the components involved in cell differentiation are more species-specific and less conserved than those involved in cell cycle regulation. However, taking as examples skeletal muscle differentiation in humans and stomatal guard cells differentiation in Arabidopsis, conserved factors that coordinate cell differentiation can be identified, namely Rb, MADS-box TFs, and TFs of the bHLH family, as well as the cyclins/CDKs protein complexes (Figure 2D) [73][74].
At post-embryonic stages, myocyte differentiation in mammals can be triggered in response to muscle damage or a specific growth-inducing stimulus. This last provokes massive proliferation of myoblasts through the hierarchical activation of several MRF (Myogenic Regulatory Factors): Myogenic Factor 5 (Myf5), myoblast determination protein 1 (MyoD), Myogenin (MyoG), and Myogenic Regulatory Factor 4B (MRF4), which are TFs that belong to the bHLH family [75][76][77]. In this process, the protein complex of MyoD with pRb, is thought to initiate differentiation, as it promotes cell cycle arrest [78][79][80][81]. Despite immunoprecipitation experiments that proved that pRb and MyoD can interact [82], later experiments using nuclear magnetic resonance allowed to determine that there is no direct protein-protein interaction between MyoD and pRb [83]. Still, MyoD does activate RB1 expression through its association with the cAMP response element-binding (CREB) TF and the coactivators p300 and P/CAF (Figure 2) [84][85].
Induction of muscle biogenesis also requires the regulation by cyclins-CDKs in association with pRb [86]. The stable repression of cyclin D1, required for cell cycle arrest during differentiation, is regulated by the joint action of MyoD and pRb through the regulation of the upstream intermediary gene Fra-1 (antigen related to FOS 1) (Figure 2B) [87][88]. Antagonistically, cyclin D1 inhibits the activity of MyoD: overexpression of cyclin D1 promotes nuclear accumulation of CDK4, that binds MyoD, preventing its interaction with DNA, and inhibiting the CDK4-dependent phosphorylation of pRb (Figure 2B) [89][90][91]. Additionally, when HDAC1 interacts with pRb, the MyoD protein can bind its target DNA regulatory sites [92][93]. In summary, there are two different protein complexes formed during different stages of muscle development: HDAC1/MyoD during proliferation and HDAC1/hypophosphorylated pRb during differentiation (Figure 2B) [92][93].
In plants, some epidermal cells undergo differentiation producing the two mature guard cells that form the stomata pores, structures that are conserved among land plants and allow them to regulate gas exchange and water loss [94][95]. Stomatal development is hierarchically regulated by the sequential activation of several TFs of the bHLH family: SPCH (SPEECHLESS), MUTE, and FAMA. These three bHLHs form heterodimers with either the bHLHs SCREAM (SCRM, also called ICE1) or SCRM2 [96][97][98], that belong to the same family of TFs that participate in muscle development in mammals (MRFs) (Figure 2D). These plant TFs orchestrate cell division events of protodermal cells, which give origin to guard cells (stomatogenesis). SPCH triggers the maturation of a protodermal cell into a meristemoid mother cell (MMC) and is also involved in the asymmetric cell division of the MMC, that results in one meristemoid cell and one larger sister cell (SLGC). The meristemoid cell exits stemness and engages in differentiation to become a guard meristemoid cell (GMC). MUTE must be expressed at this stage, to direct further differentiation of a GMC, and to ensure that this cell undergoes a single symmetric division. In addition MUTE regulates the expression of FAMA, which controls the final stages of differentiation, promoting guard cell (GC) identity acquisition and the irreversible termination of the meristematic activity of the cells (Figure 2C) [99][100][101]. AtRBR plays important roles in the regulatory network of stomata development, and its downregulation at the GMC or GC stages, induces extra divisions in differentiated GCs and the formation of aberrant stomata-in-stomatal nested structures [102][101]. In fact, AtRBR hyperphosphorylation inhibits stomatal initiation affecting the asymmetric division of protodermal cells that produces MMCs, this seems to be controlled by CDKA;1, that negatively regulates AtRBR, and regulates positively SPCH TF through phosphorylation (Figure 2C) [103][104][105]. It has also been hypothesized that AtRBR hyperphosphorylation by CDKB1;1-CYCD7;1 inhibits the AtRBR/FAMA repression complex leading to the induction of cell-cycle regulators of the GMC symmetric division event [106][107][108]. At the same time, MUTE directly upregulates FAMA and FLP; and FAMA represses cell-cycle control genes such as CDKB1;1, ensuring a single symmetric division to form GCs (Figure 2C) [109][99]. Mutation in the FAMA LxCxE sequence prevents the formation of the AtRBR/FAMA complex, making cells unable to maintain the long-term commitment to differentiate into GC, and arresting this process at the GMC stage [101][106]. Finally, it is also possible that FAMA functions at early steps of guard cell differentiation since the AtRBR/FAMA heterodimer binds to SPCH and FAMA promoters, and this complex negatively regulates the accumulation of the SPCH transcript, which is normally expressed at early stages of guard cells development (Figure 2C) [101][110].
As it can be appreciated, the differentiation processes of skeletal muscle in mammals and guard cells in Arabidopsis are both regulated by a similar set of conserved elements: pRb/RBR, bHLHs, cyclins and CDKs (Figure 2D).
In mammals and plants, many epigenetic modifier proteins interact with protein complexes that include pRb/RBR, allowing them to regulate different developmental processes.
Human pRb has been reported to interact with over 300 proteins and many such protein interactions are epigenetic modifier proteins or interact with the latter [111][112]. pRb levels decrease leads to an incomplete chromosome condensation and segregation during mitosis, as it has been observed in cancer cells; some alterations of the chromatin structure are also induced by changes in histone methylation and acetylation [113][114][115]. pRb can interact with chromatin remodeling factors, such as histone deacetylases, DNA methyltransferases, histone methyltransferases, and with complexes like SWI/SNF; and like Polycomb group (PcG), the latter is a chromatin-modifying complex that maintains repressed gene expression states and is subdivided into two main complexes: Polycomb repressive complex 1 (PRC1) and PRC2 [113][116][117][118][119].
In the case of a double strand break, pRb is necessary to form the complex of the heterodimer E2F1-pRb with TopBP1 (DNA topoisomerase 2-binding protein 1) [120]. TopBP1 is a protein that interacts with Topoisomerase 2β (Top2β) and with other proteins that participate in DNA replication and in the maintenance of the DNA integrity and genome stability [115][121]. In addition, the protein complex E2F1-pRb-TopBP1 interacts with BRG1 (also known as ATP-dependent chromatin remodeler SMARCA4) which is a member of the SWI/SNF complex that is necessary to reduce nucleosome density at injury sites, allowing DNA end resection and reparation by homologous recombination (HR) [120][122]. pRb interacts with the tumor suppressor BRCA1 (Breast cancer 1), which is also involved in DNA repair via Homologous Recombination. The BRCA1-pRb complex interacts with histone deacetylases (HDAC1/2) and with topoisomerase 2β (Top2β) to regulate DNA stability [123][124].
In plants, there are also numerous reports of RBR interactions with epigenetic modifiers, which are important in the regulation of different developmental processes [125]. In Arabidopsis, like in animals, it has been shown that PRC2, a subcomplex of PcG, participates together with AtRBR in the establishment of the H3K27me3 mark during differentiation and development of the female and male gametophytes, in leaf development and during the establishment of stoma cell lineages. In these three processes, AtRBR associates with components of the PRC2 repressor complex such as MULTICOPYSUPPRESSOR OF IRA1 (MSI1), FERTILIZATION INDEPENDENT ENDOSPERM (FIE), VERNALIZATION 2 (VRN2), and CURLY LEAF (CLF), a gene orthologous to EZH2 from humans [126][127]. In addition, AtRBR together with MSI1 directly represses the expression of the DNA methylase METHYLTRANSFERASE 1 (MET1), that maintains DNA methylation during DNA replication and regulates gene imprinting. The repression of MET1 by this complex allows the transcriptional activation of FERTILIZATION INDEPENDENT SEED 2 (FIS2) and FLOWERING WAGENINGEN (FWA), that are important for female gametogenesis [127][128][129]. In plant embryos, the PRC2 complex with AtRBR directly binds and deposits the H2K27m3 mark on different embryonic genes, leading to their repression and subsequent seed germination [130][131].
AtRBR also appears to regulate DNA repair in several conditions. TOP1α is critical to ensure genome integrity and survival of root stele stem cells, as the loss of function of TOP1α triggers DNA double-strand breaks and cell death in these cells; in the root, TOP1α is downregulated by AtRBR [132]. Although the participation of AtRBR and Top1α in the shoot meristem have not yet been studied, TOP1α participates with the PRC2 complex in the repression of the WUS locus [133][132][134].
AtRBR also is recruited to damaged DNA sites, along with E2Fa and AtBRCA1 and helps to maintain the integrity of the root meristem. Furthermore, similar to what is observed for animals for BRCA1 and pRb [116][135], AtRBR and AtBRCA1 have been shown to physically interact when cells are damaged [136]. In addition, E2Fa is required for AtBRCA1 expression, when genotoxic stress is induced [137]. Finally, analysis of chromosome sites to which AtRBR physically binds, show that this protein not only targets gene regulatory sequences, but also transposons, especially Miniature Inverted-repeat Transposable Elements (MITEs) [57].